![]() NEET 2016 – 1
NEET 2016 – 1
|
Section |
Questions |
Marks |
|
Physics |
45 Questions (1 – 45) |
180 |
|
Chemistry |
45 Questions (46 – 90) |
180 |
|
Biology |
90 Questions (91 – 180) |
360 |
![]() Q. 1 From a disc of radius R and mass M. a circular hole diameter R whose rim passes through the centre is cut. What the moment of inertia of the remaining part of the disc about perpendicular axis & passing through the centre?
Q. 1 From a disc of radius R and mass M. a circular hole diameter R whose rim passes through the centre is cut. What the moment of inertia of the remaining part of the disc about perpendicular axis & passing through the centre?
A. 15 MR²/32
B. 13 MR²/32
C. 11 MR²/32
D. 9 MR²/32
![]() Q. 2 A square loop ABCD carrying a current i, is placed near and coplanar with a long straight conductor XY carrying a current I. The net force on the loop will be:
Q. 2 A square loop ABCD carrying a current i, is placed near and coplanar with a long straight conductor XY carrying a current I. The net force on the loop will be:
A. 2μ₀li/3π
B. μ₀li/2π
C. 2μ₀liL/3π
D. μ₀liL/2π
![]() Q. 3 The magnetic suscepetibility is negative for
Q. 3 The magnetic suscepetibility is negative for
A. diamagnetic material only
B. paramagnetic material only
C. ferromagnetic material only
D. Paramagnetic and ferromagnetic materials
![]() Q. 4 A siren emitting a sound of frequency 800 Hz moves away from an observer towards a cliff at a a speed of 15 ms⁻¹ .Then , the frequency of sound that the observer hears in the echo reflected from the cliff is :
Q. 4 A siren emitting a sound of frequency 800 Hz moves away from an observer towards a cliff at a a speed of 15 ms⁻¹ .Then , the frequency of sound that the observer hears in the echo reflected from the cliff is :
(Take velocity of sound in air = 330 ms⁻¹)
A. 765 Hz
B. 800 Hz
C. 838 Hz
D. 885 Hz
![]() Q. 5 A capacitor of 2 μF is charged as shown in the diagram. When the switch S is turn to position 2, the percentage of its stored energy dissipated is:
Q. 5 A capacitor of 2 μF is charged as shown in the diagram. When the switch S is turn to position 2, the percentage of its stored energy dissipated is:
A. 0%
B. 20%
C. 75%
D. 80%
![]() Q. 6 In a diffraction pattern due to a single slit of width ‘a’, the first minimum is observed at an angle 30° when light of wavelength 5000 Å is incident on the slit. The first secondary maximum is observed at an angle of:
Q. 6 In a diffraction pattern due to a single slit of width ‘a’, the first minimum is observed at an angle 30° when light of wavelength 5000 Å is incident on the slit. The first secondary maximum is observed at an angle of:
A. sin⁻¹(1/4)
B. sin⁻¹(2/3)
C. sin⁻¹(1/2)
D. sin⁻¹(3/4)
![]() Q. 7 At what height from the surface of earth the gravitation potential and the value of g are -5.4×10⁷ J kg⁻² and 6.0 ms⁻² respectively? Take the radius of earth as 6400 km:
Q. 7 At what height from the surface of earth the gravitation potential and the value of g are -5.4×10⁷ J kg⁻² and 6.0 ms⁻² respectively? Take the radius of earth as 6400 km:
A. 2600 km
B. 1600 km
C. 1400 km
D. 2000 km
![]() Q. 8 Out of the following options which one can be used to produce a propagating
Q. 8 Out of the following options which one can be used to produce a propagating
electromagnetic wave?
A. A charge moving at constant velocity
B. A stationary charge
C. A chargeless particle
D. An accelerating charge
![]() Q. 9 Two identical charged spheres suspended from a common point by two massless strings of lengths l, are initially at a distance d (d << l) apart because of their mutual repulsion. The charges begin to leak from the both the sphere at a constant rate. As a result, the sphere approach each other with a velocity v. Then v varies as a function of the distance x between the spheres as :
Q. 9 Two identical charged spheres suspended from a common point by two massless strings of lengths l, are initially at a distance d (d << l) apart because of their mutual repulsion. The charges begin to leak from the both the sphere at a constant rate. As a result, the sphere approach each other with a velocity v. Then v varies as a function of the distance x between the spheres as :
A. v proportional to x¹/²
B. v proportional to x
C. v proportional to x⁻¹/²
D. v proportional to x⁻¹
![]() Q. 10 A uniform rope of length L and mass m₁ hangs vertically from a rigid support. A block of mass m₂ is attached to the free end of the rope. A transverse pulse of wavelength λ1 is produced at the lower end of the rope. The wavelength of the pulse when it reaches the top of the rope is λ2. The ratio λ2/λ1 is:
Q. 10 A uniform rope of length L and mass m₁ hangs vertically from a rigid support. A block of mass m₂ is attached to the free end of the rope. A transverse pulse of wavelength λ1 is produced at the lower end of the rope. The wavelength of the pulse when it reaches the top of the rope is λ2. The ratio λ2/λ1 is:
A. √m₁/√m₂
B. √m₁+m₂/√m₂
C. √m₂/√m₁
D. √m₁+m₂/√m₁
![]() Q. 11 A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is:
Q. 11 A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is:
(Take 1 cal = 4.2 joules)
A. 2.365 W
B. 23.65 W
C. 236.5 W
D. 2365 W
![]() Q. 12 An air column, closed at one end and open at the other, resonates with a turning fork when the smallest length of the column is 50 cm. The next larger length of the column resonating with the same turning fork is:
Q. 12 An air column, closed at one end and open at the other, resonates with a turning fork when the smallest length of the column is 50 cm. The next larger length of the column resonating with the same turning fork is:
A. 66.7 cm
B. 100 cm
C. 150 cm
D. 200 cm
![]() Q. 13 Consider the junction diode as ideal. The value of current flowing through AB is:
Q. 13 Consider the junction diode as ideal. The value of current flowing through AB is:
A. 0 A
B. 10⁻² A
C. 10⁻¹ A
D. 10⁻³ A
![]() Q. 14 The charge flowing through a resistance R varies with time t as Q = at – bt², where a and b are positive constants. The total heat produced in R is:
Q. 14 The charge flowing through a resistance R varies with time t as Q = at – bt², where a and b are positive constants. The total heat produced in R is:
A. a³R/6b
B. a³R/3b
C. a³R/2b
D. a³R/b
![]() Q. 15 A black body is at a temperature of 5760 K. The energy of radiation emitted by the body at wavelength 250 nm is U₁. At wavelength 500 nm is U₂ and that at 1000 nm is U₃. Wien’s constant, b = 2.88 x 10⁶ nmK. Which of the following is correct?
Q. 15 A black body is at a temperature of 5760 K. The energy of radiation emitted by the body at wavelength 250 nm is U₁. At wavelength 500 nm is U₂ and that at 1000 nm is U₃. Wien’s constant, b = 2.88 x 10⁶ nmK. Which of the following is correct?
A. u₁=0
B. u₃=0
C. u₁>u₂
D. u₂>u₁
![]() Q. 16 Coefficient of linear expansion of brass and steel rods are α1 and α2. Lengths of brass and steel rods are l₁ and l₂ respectively. If (l₂ – l₁) is maintained same at all temperatures, which one of the following relations holds good?
Q. 16 Coefficient of linear expansion of brass and steel rods are α1 and α2. Lengths of brass and steel rods are l₁ and l₂ respectively. If (l₂ – l₁) is maintained same at all temperatures, which one of the following relations holds good?
A. α₁l₂ = α₂l₁
B. α₁l₂² = α₂l₁²
C. α₁²l₂ =α₂²l₁
D. α₁l₁ = α₂l₂
![]() Q. 17 A npn transistor is connected in common emitter configuration in a given amplifier. A load resistance of 800 Ω is connected in the collector circuit and the voltage drop across it is 0.8 V. Of the current amplification factor is 0.96 and the input resistance of the circuit is 192 Ω, the voltage gain and the power gain of the amplifier will respectively be:
Q. 17 A npn transistor is connected in common emitter configuration in a given amplifier. A load resistance of 800 Ω is connected in the collector circuit and the voltage drop across it is 0.8 V. Of the current amplification factor is 0.96 and the input resistance of the circuit is 192 Ω, the voltage gain and the power gain of the amplifier will respectively be:
A. 4, 3.84
B. 3.69, 3.84
C. 4, 4
D. 4, 3.69
![]() Q. 18 The intensity of the maximum in a Young’s double slit experiment is I₀. Distance between two slits of d = 5λ, where λ is the wavelength of light used in the experiment. What will be the intensity in front of one of the slits on the screen placed at a distance D = 10d?
Q. 18 The intensity of the maximum in a Young’s double slit experiment is I₀. Distance between two slits of d = 5λ, where λ is the wavelength of light used in the experiment. What will be the intensity in front of one of the slits on the screen placed at a distance D = 10d?
A. I₀
B. I₀/4
C. I₀
D. I₀/2
![]() Q. 19 A uniform circular disc of radius 50 cm at rest is free to turn about an axis which is perpendicular to its plane and passes through its center. It is subjected to a torque which produces a constant angular acceleration of 2.0 rad s⁻². Its net acceleration in ms⁻² at the end of 2.0 s is approximately.
Q. 19 A uniform circular disc of radius 50 cm at rest is free to turn about an axis which is perpendicular to its plane and passes through its center. It is subjected to a torque which produces a constant angular acceleration of 2.0 rad s⁻². Its net acceleration in ms⁻² at the end of 2.0 s is approximately.
A. 8.0
B. 7.0
C. 6.0
D. 3.0
![]() Q. 20 An electron of mass m and a photon have same energy E. The ratio of de-Broglie wavelengths associated with them is :
Q. 20 An electron of mass m and a photon have same energy E. The ratio of de-Broglie wavelengths associated with them is :
A. (1/c)(E/2m)¹/²
B. (E/2m)¹/²
C. c(2mE)¹/²
D. (1/E)(2m)²⁺⁽¹/ ᶜ⁾
![]() Q. 21 A disk and a sphere o same radius but different masses roll off on two inclined planes of the same altitude and lengths. Which one of the two objects gets to the bottom of the plane first?
Q. 21 A disk and a sphere o same radius but different masses roll off on two inclined planes of the same altitude and lengths. Which one of the two objects gets to the bottom of the plane first?
A. Disk
B. Sphere
C. Both reach at the same time
D. Depends on their masses
![]() Q. 22 The angle of incidence for a ray light at a refracting surface of a prism is 45°. The angle of prism is 60°. If the ray suffers minimum deviation through the prism, the angle of minimum deviation and refractive index of the material of the prism respectively, are:
Q. 22 The angle of incidence for a ray light at a refracting surface of a prism is 45°. The angle of prism is 60°. If the ray suffers minimum deviation through the prism, the angle of minimum deviation and refractive index of the material of the prism respectively, are:
A. 45°1;/√2
B. 30°;√2
C. 45°;√2
D. 30°;1∕√2
![]() Q. 23 When an α – particle of mass ‘m’ moving with velocity ‘’ bombards on a heavy nucleus of charge ‘Ze’, its distance of closet approach from the nucleus depends on m as:
Q. 23 When an α – particle of mass ‘m’ moving with velocity ‘’ bombards on a heavy nucleus of charge ‘Ze’, its distance of closet approach from the nucleus depends on m as:
A. 1/m
B. 1/√m
C. 1/m²
D. M
![]() Q. 24 A particle of mass 10 g moves along a circle of radius 6.4 c with a constant tangential acceleration. What is the magnitude of this acceleration if the kinetic energy of the particle becomes equal to 8 x 10⁻⁴ J by the end of the second revolution after the beginning of the motion?
Q. 24 A particle of mass 10 g moves along a circle of radius 6.4 c with a constant tangential acceleration. What is the magnitude of this acceleration if the kinetic energy of the particle becomes equal to 8 x 10⁻⁴ J by the end of the second revolution after the beginning of the motion?
A. 0.1 m/s²
B. 0.15 m/s²
C. 0.18 m/s²
D. 0.2 m/s²
![]() Q. 25 The molecules of a given mass of a gas have r.m.s. velocity of 200 ms⁻¹ at 27 °C and 1.0 x 10⁵ Nm⁻² pressure. When the temperature and pressure of the gas respectively, 127 °C and 0.05 x 10⁵ Nm⁻², the r.m.s. velocity of its molecules in ms⁻¹ is:
Q. 25 The molecules of a given mass of a gas have r.m.s. velocity of 200 ms⁻¹ at 27 °C and 1.0 x 10⁵ Nm⁻² pressure. When the temperature and pressure of the gas respectively, 127 °C and 0.05 x 10⁵ Nm⁻², the r.m.s. velocity of its molecules in ms⁻¹ is:
A. 100√2
B. 400/√3
C. 100√2/3
D. 100/3
![]() Q. 26 A long straight wire of radius a carries a steady current I. The current is uniformly distributed over its cross–section. The ratio of the magnetic fields B and B’, at radial distances a/2 and 2a respectively, from the axis of the wire is:
Q. 26 A long straight wire of radius a carries a steady current I. The current is uniformly distributed over its cross–section. The ratio of the magnetic fields B and B’, at radial distances a/2 and 2a respectively, from the axis of the wire is:
A. 1/4
B. 1/2
C. 1
D. 4
![]() Q. 27 A particle moves so that its position vector is given by r = cosωt ˆx + sin ωtyˆ . Where ω is a constant. Which of the following is true?
Q. 27 A particle moves so that its position vector is given by r = cosωt ˆx + sin ωtyˆ . Where ω is a constant. Which of the following is true?
A. Velocity and acceleration both are perpendicular to r
B. Velocity and acceleration both are parallel to r
C. Velocity is perpendicular to r and acceleration is directed towards the origin.
D. Velocity is perpendicular to r and acceleration is directed away from the origin.
![]() Q. 28 What is the minimum velocity with which a body of mass m must enter a vertical loop of radius R so that it can complete the loop?
Q. 28 What is the minimum velocity with which a body of mass m must enter a vertical loop of radius R so that it can complete the loop?
A. √gR
B. √2gR
C. √3gR
D. √5gR
![]() Q. 29 When a metallic surface is illuminated with radiation of wavelength λ, the stopping potential is V. If the same surface is illuminated with radiation of wavelength 2λ, the stopping potential is V/4 . The threshold wavelength for the metallic surface is:
Q. 29 When a metallic surface is illuminated with radiation of wavelength λ, the stopping potential is V. If the same surface is illuminated with radiation of wavelength 2λ, the stopping potential is V/4 . The threshold wavelength for the metallic surface is:
A. 4 λ
B. 5 λ
C. 5λ/2
D. 3λ
![]() Q. 30 A gas is compressed is isothermally to half its initial volume. The same gas is compressed separately through and adiabatic process until its volume is again reduced to half. Then:
Q. 30 A gas is compressed is isothermally to half its initial volume. The same gas is compressed separately through and adiabatic process until its volume is again reduced to half. Then:
A. Compressing the gas isothermally will require more work to be done.
B. Compressing the gas through adiabatic process will require more work to be done
C. Compressing the gas isothermally of adiabatically will require the same amount or work
D. Which of the case (wheather compression through isothermal or through adiabatic process) requires more work will depend upon the atomicty of the gas.
![]() Q. 31 A potentiometer wire is 100 cm long and a constant potential difference is maintained across it. Two cells are connected in series first to support one another and then in opposite direction. The balance points are obtained at 50 cm and 10 cm from the positive end of the wire in the two cases. The ratio of emf’s is
Q. 31 A potentiometer wire is 100 cm long and a constant potential difference is maintained across it. Two cells are connected in series first to support one another and then in opposite direction. The balance points are obtained at 50 cm and 10 cm from the positive end of the wire in the two cases. The ratio of emf’s is
A. 5:1
B. 5:4
C. 3:4
D. 3:2
![]() Q. 32 A astronomical telescope has objective and eyepiece of focal lengths 40 cm and 4 cm respectively. To view an object 200 cm away from the objective, the lenses must be separated by a distance
Q. 32 A astronomical telescope has objective and eyepiece of focal lengths 40 cm and 4 cm respectively. To view an object 200 cm away from the objective, the lenses must be separated by a distance
A. 37.3 cm
B. 46.0 cm
C. 50.0 cm
D. 54.0 cm
![]() Q. 33 Two non-mixing liquids of densities ρ and nρ (n > 1) are put in a container. The height of each liquids is h. A solid cylinder of length L and density d is put in this container. The cylinder floats with its axis vertical and length pL (p < 1) in the denser liquids. The density d is equal to
Q. 33 Two non-mixing liquids of densities ρ and nρ (n > 1) are put in a container. The height of each liquids is h. A solid cylinder of length L and density d is put in this container. The cylinder floats with its axis vertical and length pL (p < 1) in the denser liquids. The density d is equal to
A. {1+(n+1)p}p
B. {2+(n+1)p}p
C. {2+{n-1}p}p
D. {1+(n-1)p}p
![]() Q. 34 To get output 1 for the following circuit, the correct choice for the input is:
Q. 34 To get output 1 for the following circuit, the correct choice for the input is:
A. A = 0, B = 1, C = 0
B. A = 1, B = 0, C = 0
C. A = 1, B = 1, C = 0
D. A = 1, B = 0, C = 1
![]() Q. 35 A piece of ice falls from a height h so that it melts completely. Only one – quarter of the heat produced is absorbed by the ice and all the energy of ice gets converted into heat during its fall. The value of h is: [Latent heat of ice is 3.4 x 10⁵ J/kg and g = 10 N/kg]
Q. 35 A piece of ice falls from a height h so that it melts completely. Only one – quarter of the heat produced is absorbed by the ice and all the energy of ice gets converted into heat during its fall. The value of h is: [Latent heat of ice is 3.4 x 10⁵ J/kg and g = 10 N/kg]
A. 34km
B. 544 km
C. 136 km
D. 68 km
![]() Q. 36 The ratio of escape velocity of earth (ᵥ) to the escape velocity at a planet (p) whose radius and mean density are twice as that of earth is:
Q. 36 The ratio of escape velocity of earth (ᵥ) to the escape velocity at a planet (p) whose radius and mean density are twice as that of earth is:
A. 1 : 2
B. 1: 2√2
C. 1:4
D. 1: √2
![]() Q. 37 If the magnitude of sum of two vectors is equal to the magnitude of difference of the two vectors, the angle between these vectors is:
Q. 37 If the magnitude of sum of two vectors is equal to the magnitude of difference of the two vectors, the angle between these vectors is:
A. 0°
B. 90°
C. 45°
D. 180°
![]() Q. 38 Given the value of Rydberg constant is 10⁷ m⁻¹, the wave number of the last line of the Balmer series in hydrogen spectrum will be:
Q. 38 Given the value of Rydberg constant is 10⁷ m⁻¹, the wave number of the last line of the Balmer series in hydrogen spectrum will be:
A. 0.025 x 10⁴ m⁻¹
B. 0.5 x 10⁷ m⁻¹
C. 0.25 x 10⁷ m⁻¹
D. 2.5 x 10⁷ m⁻¹
![]() Q. 39 A body of mass 1 kg begins to move under the action of a time dependent force F=(2ti+3t₂ J)N, where i and J are unit along x and y axis. What power will be developed by the force at the time t?
Q. 39 A body of mass 1 kg begins to move under the action of a time dependent force F=(2ti+3t₂ J)N, where i and J are unit along x and y axis. What power will be developed by the force at the time t?
A. (2t² + 3t³) W
B. (2t² + 4t⁴) W
C. (2t³ + 3t⁴) W
D. (2t³ + 3t⁵) W
![]() Q. 40 An inductor 20 mH, a capacitor 50 μF and a resistor 40 Ω are connected in series across a source of emf V = 10 sin 340t. The power loss in A.C circuit is:
Q. 40 An inductor 20 mH, a capacitor 50 μF and a resistor 40 Ω are connected in series across a source of emf V = 10 sin 340t. The power loss in A.C circuit is:
A. 0.51 W
B. 0.67 W
C. 0.76 W
D. 0.89 W
![]() Q. 41 If the velocity of a particle is = At + Bt², where A and B are constants, then the distance travelled by it between is and 2s is:
Q. 41 If the velocity of a particle is = At + Bt², where A and B are constants, then the distance travelled by it between is and 2s is:
A. 3/2(A+4B)
B. 3/3(A+ 7B)
C. 23ᴬ⁺ᴮ
D. (A/2)+(B/3)
![]() Q. 42 A long solenoid has 1000 turns. When a current of 4A flows through it, the magnetic flux linked with each turn of the solenoid is 4 x 10⁻³ Wb. The self– inductance of the solenoid is:
Q. 42 A long solenoid has 1000 turns. When a current of 4A flows through it, the magnetic flux linked with each turn of the solenoid is 4 x 10⁻³ Wb. The self– inductance of the solenoid is:
A. 4H
B. 3H
C. 2H
D. 1H
![]() Q. 43 A small signal voltage V(t) = V₀ sin ωt is applied across an ideal capacitor C:
Q. 43 A small signal voltage V(t) = V₀ sin ωt is applied across an ideal capacitor C:
A. Current I(t), lags voltage V(t) by 90°.
B. Over a full cycle the capacitor C does not consume any energy from the voltage source.
C. Current I(t) is in phase with voltage V(t) .
D. Current I(t) leads voltage V(t) by 180°.
![]() Q. 44 Match the corresponding entries of column 1 with column 2 [where m is the magnification produced by the mirror]
Q. 44 Match the corresponding entries of column 1 with column 2 [where m is the magnification produced by the mirror]
A. A→b and c; B→b and c; C→b and d; D→a and d
B. A→a and c; B→a and d; C→a and b; D→c and d
C. A→a and d; B→b and c; C→b and d; D→b and c
D. A→c and d; B→b and d; C→b and c; D→a and d
![]() Q. 45 A car is negotiating a curved road of radius R. The road is banked at an angle θ. The coefficient of friction between the types of the car and the road is μ. The maximum safe velocity on this road is:
Q. 45 A car is negotiating a curved road of radius R. The road is banked at an angle θ. The coefficient of friction between the types of the car and the road is μ. The maximum safe velocity on this road is:
A. √((gR²)(μ+tanθ)/(1-μ tanθ))
B. √((gR)( μ+tanθ)/(1-μtanθ))
C. √((gμ+tanθ)/(R1-μtanθ))
D. √((g/R²)(μ+tanθ)/(1-μtanθ))
![]() Q. 46 Consider the molecules CH₄, NH₃ and H₂O. Which of the given statements is false?
Q. 46 Consider the molecules CH₄, NH₃ and H₂O. Which of the given statements is false?
A. The H – C – H bond angle in CH₄, the H – N – H bond angle in NH₃, and the H – O – H bond angle in H₂O are all greater than 90°.
B. The H – O – H bond angle in H₂O is larger than the H – C – H bond angle in CH₄.
C. The H – O – H bond angle in H₂O is smaller than the H – N – H bond angle in NH₃.
D. The H – C – H bond angle in CH₄ is larger than the H – N – H bond angle in NH₃.
![]() Q. 47 In the following reaction
Q. 47 In the following reaction
X and Y are :
A. X = 1 – Butyne; Y = 3- Hexyne
B. X = 2 – Butyne; Y = 3 – Hexyne
C. X = 2- Butyne ; Y = 2- Hexyne
D. X = 1- Butyne ; Y = 2 – Hexyne
![]() Q. 48 Among the following the correct order of acidity is :
Q. 48 Among the following the correct order of acidity is :
A. HClO₃ < HClO₄ < HClO₂< HClO
B. HClO < HClO₂ < HClO₃ < HClO₄
C. HClO₂< HClO < HClO₃ < HClO₄
D. HClO₄ < HClO₂ < HClO < HClO₃
![]() Q. 49 The rate of first order reaction is 0.04 mol⁻¹ s⁻¹at 10 seconds and 0.03 mol⁻¹ s⁻¹ at 20 seconds after intiation of the reaction. The half – life period of the reaction is :
Q. 49 The rate of first order reaction is 0.04 mol⁻¹ s⁻¹at 10 seconds and 0.03 mol⁻¹ s⁻¹ at 20 seconds after intiation of the reaction. The half – life period of the reaction is :
A. 24.1 s
B. 34.1 s
C. 44.1s
D. 54.1 s
![]() Q. 50 Which one of the following characteristics is associated with adsorption?
Q. 50 Which one of the following characteristics is associated with adsorption?
A. Δ G is negative but ΔH and ΔS are positive
B. ΔG, ΔH and ΔS all are negative
C. ΔG and ΔH are negative but ΔS is positive
D. ΔG and ΔS are negative butΔH is positive
![]() Q. 51 In which of the following options the order of arrangement does not agree with the variation of property indicated against it?
Q. 51 In which of the following options the order of arrangement does not agree with the variation of property indicated against it?
A. Al³⁺> Mg²⁺< Na⁺< F⁻ (increasing ionic size)
B. B < C < N < O (increasing first ionisation enthalpy)
C. I < Br < Cl < F (increasing electron gain enthalpy
D. Li < Na < K < Rb (increasing metallic radius)
![]() Q. 52 Which of the following statements is false?
Q. 52 Which of the following statements is false?
A. Mg²⁺ ions form a complex with ATP.
B. Ca²⁺ ions are important in blood clotting
C. Ca²⁺ ions are not important in maintaining the regular beating of the heart
D. Mg²⁺ ions are important in the green parts of plants.
![]() Q. 53 Which of the following statements about hydrogen is incorrect
Q. 53 Which of the following statements about hydrogen is incorrect
A. Hydrogen has three isotopes of which tritium is the most common
B. Hydrogen never acts as cation in ionic salts
C. Hydronium ion, H₃O⁺ exists freely in solution
D. Dihydrogen does not act as a oxidising agent
![]() Q. 54 The correct statement regarding a carbonyl compound with a hydrogen atom on its alphacarbon is :
Q. 54 The correct statement regarding a carbonyl compound with a hydrogen atom on its alphacarbon is :
A. A carbonyl compound with a hydrogen atom on its alpha – carbon never equilibrates with its corresponding enol.
B. A carbonyl compound with a hydrogen atom on its alpha – carbon rapidily equilibrates with its corresponding enol and this process is known as aldehyde – ketone equilibration
C. A carbonyl compound with a hydrogen atom on its alpha – carbon rapidly equilibrium with its corresponding enol and this process is known as carbonylation
D. A carbonyl compound with a hydrogen atom on its alpha – carbon rapidly equilibrates with its corresponding enol and this process is known as keto – enol tautomerism.
![]() Q. 55 MY and NY₃ two nearly insoluble salts, have te same ksp values of 6.2 x 10⁻¹³ at room temperature. Which statement would be true in regard of MY and NY₃?
Q. 55 MY and NY₃ two nearly insoluble salts, have te same ksp values of 6.2 x 10⁻¹³ at room temperature. Which statement would be true in regard of MY and NY₃?
A. The molar solubilities of MY and NY₃ in water are indetical
B. The molar solubility of MY in water is less than that of NY₃
C. The salts MY and NY₃ are more soluble in 0.5 M KY than in pure water.
D. The addition of the salt of KY to solution of MY and NY₃ will have no effect on their solubilities.
![]() Q. 56 In a protein molecule various amino acids are linked together by:
Q. 56 In a protein molecule various amino acids are linked together by:
A. α – glycosidid bond
B. β – glycosidic bond
C. Peptide bond
D. Dative bond
![]() Q. 57 Natural rubber has:
Q. 57 Natural rubber has:
A. All cis – configuration
B. All trans – configuration
C. Alternate cis – and trans – configuration
D. Random cis – and trans – configuration
![]() Q. 58 Match items of Column I with the item of Column II and assign the correct code :
Q. 58 Match items of Column I with the item of Column II and assign the correct code :
|
Column I |
Column II |
||
|
(a) |
Cyanide process |
(i) |
Ultrapure Ge |
|
(b) |
Froth floatation process |
(ii) |
Dressing of ZnS |
|
(c) |
Electrolytic reduction |
(iii) |
Extraction of Al |
|
(d) |
Zone refining |
(iv) |
Extraction of Au |
|
(v) |
Purification of Ni |
A. A (4), B (2), C(3), D(1)
B. A (2), B (3), C(1), D (5)
C. A(1), B(2), C(3), D(4)
D. A(3), B(4), C(5), D(1)
![]() Q. 59 Which one of the following statements is correct when SO₂ is passed through acidified K₂Cr₂O7 solution?
Q. 59 Which one of the following statements is correct when SO₂ is passed through acidified K₂Cr₂O7 solution?
A. The solution turns blue
B. The solution is decolorized
C. SO₂ is reduced.
D. Green Cr₂ (SO₄)₃ is formed
![]() Q. 60 The electronic configurations of Eu (Atomic No.63), Gd (Atomic No.64 and Tb (Atomic No.65) are :
Q. 60 The electronic configurations of Eu (Atomic No.63), Gd (Atomic No.64 and Tb (Atomic No.65) are :
A. [Xe]4f⁷6s², [Xe]4f⁸6s² and [Xe]4f⁸5d¹6s²
B. [Xe]4f⁶5d¹6s², [Xe]4f⁷5d¹6s² and [Xe]4f⁹6s²
C. [Xe]4f⁶5d¹6s², [Xe]4f⁷5d¹6s² and [Xe]4f⁸5d¹6s²
D. [Xe]4f⁷6s², [Xe]4f⁷5d¹6s² and [Xe]4f⁹6s²
![]() Q. 61 Two electrons occupying the same orbital are distinguished by :
Q. 61 Two electrons occupying the same orbital are distinguished by :
A. Principle quantum number
B. Magnetic quantum number
C. Azimutual qautum number
D. Spin qauntum number
![]() Q. 62 When copper is heated with cone. HNO₃ it produces:
Q. 62 When copper is heated with cone. HNO₃ it produces:
A. Cu(NO₃) and NO₂
B. Cu(NO₃) and NO
C. Cu(NO₃)₂, and NO₂
D. Cu(NO₃)₂ and N₂O
![]() Q. 63 Which of the following regents would distinguish cis-cyclopenta-1, 2-diol from the transisomer?
Q. 63 Which of the following regents would distinguish cis-cyclopenta-1, 2-diol from the transisomer?
A. Aceptone
B. Ozone
C. MnO₂
D. Aluminium isopropoxide
![]() Q. 64 The correct thermodynamic conditions for the spontaneous reaction at all temperature is :
Q. 64 The correct thermodynamic conditions for the spontaneous reaction at all temperature is :
A. H < 0 and S = 0
B. H > 0 and S < 0
C. H < 0 and S > 0
D. H < 0 and S < 0
![]() Q. 65 Lithium has a bcc structure. Its density is 530 kg m-3 and its atomic mass is 6.94g mol⁻¹. Calculate the edge length of a unit cell of Lithium metal. (Nₐ = 6.02 × 10²³ mol⁻¹)
Q. 65 Lithium has a bcc structure. Its density is 530 kg m-3 and its atomic mass is 6.94g mol⁻¹. Calculate the edge length of a unit cell of Lithium metal. (Nₐ = 6.02 × 10²³ mol⁻¹)
A. 154 pm
B. 352 pm
C. 527 pm
D. 264 pm
![]() Q. 66 Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules ?
Q. 66 Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules ?
A. (1)I₂ > Br₂ > Cl₂ > F₂
B. Cl₂ > Br₂ > F₂ > I₂
C. Br₂ > I₂ > F₂ > Cl₂
D. F₂ > Cl₂ > Br₂ > I₂
![]() Q. 67 Which of the following is an analgesic ?
Q. 67 Which of the following is an analgesic ?
A. Novalgin
B. Penicllin
C. Streptomycin
D. Chloromycetion
![]() Q. 68 Equal moles of hydrogen and oxygen gases are placed in a container with a pin-hole through which both can escape. What fraction of the oxygen escapes in the time required for one-half of the hydrogen to escape?
Q. 68 Equal moles of hydrogen and oxygen gases are placed in a container with a pin-hole through which both can escape. What fraction of the oxygen escapes in the time required for one-half of the hydrogen to escape?
A. 1/8
B. 1/4
C. 3/8
D. 1/2
![]() Q. 69 Consider the nitration of benezene using mixed conc. H₂SO₄ and HNO₃. If a large amount of KHSO₄ is added to the mixture, the rate of nitration will be:
Q. 69 Consider the nitration of benezene using mixed conc. H₂SO₄ and HNO₃. If a large amount of KHSO₄ is added to the mixture, the rate of nitration will be:
A. faster
B. slower
C. unchanged
D. doubled
![]() Q. 70 Predict the correct order among the following:
Q. 70 Predict the correct order among the following:
A. lone pair – lone pair > lone pair – bond pair > bond pair – bond pair
B. lone pair – lone pair > bond pair – bond pair > lone pair – bond pair
C. bond pair – bond pair > lone pair – bond pair > lone pair – lone pair
D. lone pair – bond pair > bond pair – bond pair > lone pair – lone pair
![]() Q. 71 The product obtained as a result of a reaction of nitrogen with CaC₂ is :
Q. 71 The product obtained as a result of a reaction of nitrogen with CaC₂ is :
A. Ca(CN)₂
B. CaCN
C. CaCN₃
D. Ca₂CN
![]() Q. 72 Consider the following liquid — vapor equilibrium.
Q. 72 Consider the following liquid — vapor equilibrium.
Liquid ⇔ Vapor
Which of the following relations is correct?
A. dlnG/dT² =Δ Hᵥ/RT²
B. dlnP/dT = -ΔHᵥ/RT
C. dlnP/dT² = -ΔHᵥ/T²
D. dlnP/dT = ΔHᵥ/RT²
![]() Q. 73 Match the compounds given in set 1 with the hybridization and shape given in set 2 and mark the correct option.
Q. 73 Match the compounds given in set 1 with the hybridization and shape given in set 2 and mark the correct option.
Set 1
(a) XeF6
(b) XeO3
(c) XeOF4
(d) XeF4
Set 2
(i) distorted octahedral
(ii) square planar
(iii) pyramidal
(iv) square pyramidal
A. (a) – (i) (b) – (iii) (c) – (iv) (d) – (ii)
B. (a) – (i) (b) – (ii) (c) – (iv) (d) – (iii)
C. (a) – (iv) (b) – (ii) (c) – (i) (d) – (ii)
D. (a) – (iv) (b) – (i) (c) – (ii) (d) – (iii)
![]() Q. 74 Which of the following has longest C – O bond length? (Free C – O bond length in CO is 1.28Å.)
Q. 74 Which of the following has longest C – O bond length? (Free C – O bond length in CO is 1.28Å.)
A. Ni(CO)₄
B. [Co(CO)4]⊖
C. [Fe(CO)₄]²⁻
D. [Mn(CO)₆]⁺
![]() Q. 75 The pressure of H2 required to make the potential of H₂ –electrode zero in pure water at 298 K is:
Q. 75 The pressure of H2 required to make the potential of H₂ –electrode zero in pure water at 298 K is:
A. 10⁻¹⁴ atm
B. 10⁻¹² atm
C. 10⁻¹⁰ atm
D. 10⁻⁴ atm
![]() Q. 76 The addition of a catalyst during a chemical reaction alters which of the following quantities?
Q. 76 The addition of a catalyst during a chemical reaction alters which of the following quantities?
A. Entropy
B. Internal Energy
C. Enthalpy
D. Activation Energy
![]() Q. 77 The ionic radii of A⁺ and B⁻ ions are 0.98 × 10⁻¹⁰ m and 1.81 × 10⁻¹⁰ m. The coordination number of each ion in AB is:
Q. 77 The ionic radii of A⁺ and B⁻ ions are 0.98 × 10⁻¹⁰ m and 1.81 × 10⁻¹⁰ m. The coordination number of each ion in AB is:
A. 6
B. 4
C. 8
D. 2
![]() Q. 78 Which is the correct statement for the given acids?
Q. 78 Which is the correct statement for the given acids?
A. Phosphinic acid is a diprotic acid while Phosphonic acid is a monoprotic acid.
B. Phosphinic acid is a monoprotic acid while Phosphonic acid is a diprotic acid.
C. Both are triprotic acids.
D. Both are diprotic acids.
![]() Q. 79 Fog is colloidal solution of:
Q. 79 Fog is colloidal solution of:
A. Liquid in gas
B. Gas in liquid
C. Solid in gas
D. Gas in gas
![]() Q. 80 Which of the following statements about the composition of the vapour over an ideal 1: 1 molar mixture of benzene and toluene is correct? Assume that the temperature is constant at 25˚C. (Given, Vapour Pressure Data at 25˚C, benzene = 12.8 kPa, toluene = 3.85 kPa)
Q. 80 Which of the following statements about the composition of the vapour over an ideal 1: 1 molar mixture of benzene and toluene is correct? Assume that the temperature is constant at 25˚C. (Given, Vapour Pressure Data at 25˚C, benzene = 12.8 kPa, toluene = 3.85 kPa)
A. The vapour will contain a higher percentage of benzene.
B. The vapour will contain a higher percentage of toluene.
C. The vapour will contain equal amounts of benzene and toluene.
D. Not enough information is given to make a prediction.
![]() Q. 81 The correct statement regarding the comparison of staggered and eclipsed conformations of ethane, is:
Q. 81 The correct statement regarding the comparison of staggered and eclipsed conformations of ethane, is:
A. The staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has torsional strain.
B. The eclipsed conformation of ethane is more stable than staggered conformation, because eclipsed conformation has no torsional strain.
C. The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has torsional strain.
D. The staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain.
![]() Q. 82 The following reaction can be classified as:
Q. 82 The following reaction can be classified as:
A. Williamson ether synthesis reaction
B. Alcohol formation reaction
C. Dehydration reaction
D. Williamson alcohol synthesis reaction
![]() Q. 83 The product formed by the reaction of an aldehyde with a primary amine is:
Q. 83 The product formed by the reaction of an aldehyde with a primary amine is:
A. Schiff base
B. Ketone
C. Carboxylic acid
D. Aromatic acid
![]() Q. 84 Which of the following biphenyl is optically active?
Q. 84 Which of the following biphenyl is optically active?
A. (a)
B. (b)
C. (c)
D. (d)
![]() Q. 85 Which of the following statements is correct?
Q. 85 Which of the following statements is correct?
A. (a) and (b) are elimination reactions and (c) is addition reaction.
B. (a) is elimination, (b) is substitution and (c) is addition reaction.
C. (a) is elimination, (b) and (c) are substitution reactions.
D. (a) is substitution, (b) and (c) are addition reactions.
![]() Q. 86 At 100˚C the vapour pressure of a solution of 6.5 g of a solute in 100 g water is 732mm. If Kb = 0.52, the boiling point of this solution will be:
Q. 86 At 100˚C the vapour pressure of a solution of 6.5 g of a solute in 100 g water is 732mm. If Kb = 0.52, the boiling point of this solution will be:
A. 101˚C
B. 100˚C
C. 102˚C
D. 103˚C
![]() Q. 87 The correct statement regarding RNA and DNA, respectively is :
Q. 87 The correct statement regarding RNA and DNA, respectively is :
A. The sugar component in RNA is arabinose and the sugar component in DNA is 2’- deoxyribose.
B. The sugar component in RNA is ribose and the sugar component in DNA is 2’- deoxyribose.
C. The sugar component in RNA is arabinose and the sugar component in DNA is ribose.
D. The sugar component in RNA is 2’- deoxyribose and the sugar component in DNA is arabinose.
![]() Q. 88 The correct statement regarding the basicity of arylamines is :
Q. 88 The correct statement regarding the basicity of arylamines is :
A. Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring π electron system.
B. Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring π electron system.
C. Arylamines are generally more basic than alkylamines because of aryl group.
D. Arylamines are generally more basic than alkylamines, because the nitrogen atom in arylamines is sp-hybridized.
![]() Q. 89 Which one given below is a non-reducing sugar?
Q. 89 Which one given below is a non-reducing sugar?
A. Maltose
B. Lactose
C. Glucose
D. Sucrose
![]() Q. 90 The pair of electron in the given carbanion, CH₃CH ≡ C^⊖, is present in which of the following orbitals?
Q. 90 The pair of electron in the given carbanion, CH₃CH ≡ C^⊖, is present in which of the following orbitals?
A. 2p
B. sp³
C. sp²
D. sp
![]() Q. 91 Gause’s principle of competitive exclusion states that:
Q. 91 Gause’s principle of competitive exclusion states that:
A. More abundant species will exclude the less abundant species through competition.
B. Competition for the same resources excludes species having different food preferences.
C. No two species can occupy the same niche indefinitely for the same limiting resources.
D. Larger organisms exclude smaller ones through competition.
![]() Q. 92 The two polypeptides of human insulin are linked together by:
Q. 92 The two polypeptides of human insulin are linked together by:
A. Hydrogen bonds
B. Phosphodiester bond
C. Covalent bond
D. Disulphide bridges
![]() Q. 93 The coconut water from tender coconut represents:
Q. 93 The coconut water from tender coconut represents:
A. Endocarp
B. Fleshy mesocarp
C. Free nuclear proembryo
D. Free nuclear endosperm
![]() Q. 94 Which of the following statements is wrong for viroids?
Q. 94 Which of the following statements is wrong for viroids?
A. They lack a protein coat
B. They are smaller than viruses
C. They cause infections
D. Their RNA is of high molecular weight
![]() Q. 95 Which of the following features is not present in the Phylum-Arthropoda?
Q. 95 Which of the following features is not present in the Phylum-Arthropoda?
A. Chitinous exoskeleton
B. Metameric segmentation
C. Parapodia
D. Jointed appendages
![]() Q. 96 Which of the following most appropriately describes haemophilia?
Q. 96 Which of the following most appropriately describes haemophilia?
A. Recessive gene disorder
B. X-linked recessive gene disorder
C. Chromosomal disorder
D. Dominant gene disorder
![]() Q. 97 Emerson’s enhancement effect and Red drop have been instrumental in the discovery of:
Q. 97 Emerson’s enhancement effect and Red drop have been instrumental in the discovery of:
A. Photophosphorylation and non-cyclic electron transport
B. Two photosystems operating simultaneously
C. Photophosphorylation and cyclic electron transport
D. Oxidative phosphorylation
![]() Q. 98 In which of the following, all three are macronutrients?
Q. 98 In which of the following, all three are macronutrients?
A. Boron, zinc, manganese
B. Iron, copper, molybdenum
C. Molybdenum, magnesium, manganese
D. Nitrogen, nickel, phosphorus
![]() Q. 99 Name the chronic respiratory disorder caused mainly by cigarette smoking:
Q. 99 Name the chronic respiratory disorder caused mainly by cigarette smoking:
A. Emphysema
B. Asthma
C. Respiratory acidosis
D. Respiratory alkalosis
![]() Q. 100 A system of rotating crops with legume or grass pasture to improve soil structure and fertility is called:
Q. 100 A system of rotating crops with legume or grass pasture to improve soil structure and fertility is called:
A. Ley farming
B. Contour farming
C. Strip farming
D. Shifting agriculture
![]() Q. 101 Mitochondria and chloroplast are:
Q. 101 Mitochondria and chloroplast are:
(a) semi-autonomous organelles.
(b) formed by division of pre-existing organelles and they contain DNA but lack protein synthesizing machinery.
Which one of the following options is correct?
A. Both (a) and (b) are correct.
B. (b) is true but (a) is false.
C. (a) is true but (b) is false.
D. Both (a) and (b) are false.
![]() Q. 102 In context of Amniocentesis, which of the following statement is incorrect?
Q. 102 In context of Amniocentesis, which of the following statement is incorrect?
A. It is usually done when a woman is between 14 – 16 weeks pregnant.
B. It is used for prenatal sex determination.
C. It can be used for detection of Down syndrome.
D. It can be used for detection of Cleft palate.
![]() Q. 103 In a chloroplast the highest number of protons are found in 2
Q. 103 In a chloroplast the highest number of protons are found in 2
A. Stroma
B. Lumen of thylakoids
C. Inter membrane space
D. Antennae complex
![]() Q. 104 Photosensitive compound in human eye is made up of :
Q. 104 Photosensitive compound in human eye is made up of :
A. Guanosine and Retinol
B. Opsin and Retinal
C. Opsin and Retinol
D. Transducin and Retinene
![]() Q. 105 Spindle fibres attach on to:
Q. 105 Spindle fibres attach on to:
A. Telomere of the chromosome
B. Kinetochore of the chromosome
C. Centromere of the chromosome
D. Kinetosome of the chromosome
![]() Q. 106 Which is the National Aquatic Animal of India?
Q. 106 Which is the National Aquatic Animal of India?
A. Gangetic shark
B. River dolphin
C. Blue whale
D. Sea – horse
![]() Q. 107 Which of the following is required as inducer(s) for the expression of Lac operon?
Q. 107 Which of the following is required as inducer(s) for the expression of Lac operon?
A. glucose
B. galactose
C. lactose
D. lactose and galactose
![]() Q. 108 Which of the following pairs of hormones are not antagonistic (having opposite effects) to each other?
Q. 108 Which of the following pairs of hormones are not antagonistic (having opposite effects) to each other?
A. Parathormone – Calcitonin
B. Insulin – Glucagon
C. Aldosterone – Atrial Natriuretic Factor
D. Relaxin – lnhibin
![]() Q. 109 Microtubules are the constituents of:
Q. 109 Microtubules are the constituents of:
A. Cilia, Flagella and Peroxisomes
B. Spindle fibres, Centrioles and Cilia
C. Centrioles, Spindle fibres and Chromatin
D. Centrosome, Nucleosome and Centrioles
![]() Q. 110 A complex of ribosomes attached to a single strand of RNA is known as:
Q. 110 A complex of ribosomes attached to a single strand of RNA is known as:
A. Polysome
B. Polymer
C. Polypeptide
D. Okazaki fragment
![]() Q. 111 Fertilization in humans is practically feasible only if:
Q. 111 Fertilization in humans is practically feasible only if:
A. the sperms are transported into vagina just after the release of ovum in fallopian tube.
B. the ovum and sperms are transported simultaneously to ampullary – isthmic junction of the fallopian tube.
C. the ovum and sperms are transported simultaneously to ampullary – isthmic junction of the cervix.
D. the sperms are transported into cervix within 48 hrs of release of ovum in uterus.
![]() Q. 112 Asthma may be attributed to:
Q. 112 Asthma may be attributed to:
A. bacterial infection of the lungs
B. allergic reaction of the mast cells in the lungs
C. inflammation of the trachea
D. accumulation of fluid in the lungs
![]() Q. 113 The Avena curvature is used for bioassay of:
Q. 113 The Avena curvature is used for bioassay of:
A. ABA
B. GA₃
C. IAA
D. Ethylene
![]() Q. 114 The standard petal of a papilionaceous corolla is also called:
Q. 114 The standard petal of a papilionaceous corolla is also called:
A. Carina
B. Pappus
C. Vexillum
D. Corona
![]() Q. 115 Tricarpellary, syncarpous gynoecium is found in flowers of:
Q. 115 Tricarpellary, syncarpous gynoecium is found in flowers of:
A. Liliaceae
B. Solanaceae
C. Fabaceae
D. Poaceae
![]() Q. 116 One of the major components of cell wall of most fungi is :
Q. 116 One of the major components of cell wall of most fungi is :
A. Chitin
B. Peptidoglycan
C. Cellulose
D. Hemicellulose
![]() Q. 117 Select the incorrect statement :
Q. 117 Select the incorrect statement :
A. FSH stimulates the sertoli cells which help in spermiogenesis.
B. LH triggers ovulation in ovary.
C. LH and FSH decrease gradually during the follicular phase.
D. LH triggers secretion of androgens from the Leydig cells.
![]() Q. 118 In meiosis crossing over is initiated at:
Q. 118 In meiosis crossing over is initiated at:
A. Pachytene
B. Leptotene
C. Zygotene
D. Diplotene
![]() Q. 119 A tall true breeding garden pea plant is crossed with a dwarf true breeding garden pea plant. When the F1 plants were selfed the resulting genotypes were in the ratio of:
Q. 119 A tall true breeding garden pea plant is crossed with a dwarf true breeding garden pea plant. When the F1 plants were selfed the resulting genotypes were in the ratio of:
A. 1 : 2 : 1 :: Tall homozygous : Tall heterozygous : Dwarf
B. 1 : 2 : 1 :: Tall heterozygous : Tall homozygous : Dwarf
C. 3 : 1 :: Tall : Dwarf
D. 3 : 1 :: Dwarf : Tall
![]() Q. 120 Which of the following is the most important cause of animals and plants being driven to extinction?
Q. 120 Which of the following is the most important cause of animals and plants being driven to extinction?
A. Over – exploitation
B. Alien species invasion
C. Habitat loss and fragmentation
D. Co-extinctions
![]() Q. 121 Which one of the following is a characteristic feature of cropland ecosystem?
Q. 121 Which one of the following is a characteristic feature of cropland ecosystem?
A. Absence of soil organisms
B. Least genetic diversity
C. Absence of weeds
D. Ecological succession
![]() Q. 122 Changes in GnRH pulse frequency in females is controlled by circulating levels of:
Q. 122 Changes in GnRH pulse frequency in females is controlled by circulating levels of:
A. estrogen and progesterone
B. estrogen and inhibin
C. progesterone only
D. progesterone and inhibin
![]() Q. 123 Which of the following is not a feature of the plasmids?
Q. 123 Which of the following is not a feature of the plasmids?
A. Independent replication
B. Circular structure
C. Transferable
D. Single-stranded
![]() Q. 124 Which of the following features is not present in Periplaneta americana?
Q. 124 Which of the following features is not present in Periplaneta americana?
A. Schizocoelom as body cavity
B. Indeterminate and radial cleavage during embryonic development
C. Exoskeleton composed of N-acetylglucosamine
D. Metamerically segmented body
![]() Q. 125 In higher vertebrates, the immune system can distinguish self-cells and non-self. If this property is lost due to genetic abnormality and it attacks self-cells, then it leads to:
Q. 125 In higher vertebrates, the immune system can distinguish self-cells and non-self. If this property is lost due to genetic abnormality and it attacks self-cells, then it leads to:
A. Allergic response
B. Graft rejection
C. Auto-immune disease
D. Active immunity
![]() Q. 126 Match the terms in Set 1 with their description in Set2 and choose the correct option:
Q. 126 Match the terms in Set 1 with their description in Set2 and choose the correct option:
Set 1
(a) Dominance
(b) Codominance
(c) Pleiotropy
(d) Polygenic inheritance
Set 2
(i) Many genes govern a single character
(ii) In a heterozygous organism only one allele expresses itself
(iii) In a heterozygous organism both alleles express themselves fully
(iv) A single gene influences many characters
A. (a) – (ii) (b) – (i) (c) – (iv) (d) – (iii)
B. (a) – (ii) (b) – (iii) (c) – (iv) (d) – (i)
C. (a) – (iv) (b) – (i) (c) – (ii) (d) – (iii)
D. (a) – (iv) (b) – (iii) (c) – (i) (d) – (ii)
![]() Q. 127 Joint Forest Management Concept was introduced in India during:
Q. 127 Joint Forest Management Concept was introduced in India during:
A. 1960s
B. 1970s
C. 1980s
D. 1990s
![]() Q. 128 Pick out the correct statements:
Q. 128 Pick out the correct statements:
(a) Haemophilia is a sex-linked recessive disease.
(b) Down’s syndrome is due to aneuploidy.
(c) Phenylketonuria is an autosomal recessive gene disorder.
(d) Sickle cell anaemia is an X-linked recessive gene disorder.
A. (a) and (d) are correct.
B. (b) and (d) are correct.
C. (a), (c) and (d) are correct.
D. (a), (b) and (c) are correct.
![]() Q. 129 Which one of the following statements is wrong?
Q. 129 Which one of the following statements is wrong?
A. Cyanobacteria are also called blue-green algae.
B. Golden algae are also called desmids.
C. Eubacteria are also called false bacteria.
D. Phycomycetes are also called algal fungi.
![]() Q. 130 Proximal end of the filament of stamen is attached to the:
Q. 130 Proximal end of the filament of stamen is attached to the:
A. Anther
B. Connective
C. Placenta
D. Thalamus or petal
![]() Q. 131 Which of the following approaches does not give the defined action of contraceptive?
Q. 131 Which of the following approaches does not give the defined action of contraceptive?
A. Barrier methods – prevent fertilization
B. Intra uterine devices – increase phagocytosis of sperms, suppress sperm motility and fertilizing capacity of sperms
C. Hormonal contraceptives – Prevent/ retard entry of sperms, prevent ovulation and fertilization
D. Vasectomy – prevents spermatogenesis
![]() Q. 132 The taq polymerase enzyme is obtained from:
Q. 132 The taq polymerase enzyme is obtained from:
A. Thermus aquaticus
B. Thiobacillus ferroxidans
C. Bacillus subtilis
D. Pseudomonas putida
![]() Q. 133 Identify the correct statement on ’inhibin’:
Q. 133 Identify the correct statement on ’inhibin’:
A. Inhibits the secretion of LH, FSH and Prolactin.
B. Is produced by granulose cells in ovary and inhibits the secretion of FSH.
C. Is produced by granulose cells in ovary and inhibits the secretion of LH.
D. Is produced by nurse cells in testes and inhibits the secretion of LH.
![]() Q. 134 Which part of the tobacco plant is infected by Meloidogyne incognita?
Q. 134 Which part of the tobacco plant is infected by Meloidogyne incognita?
A. Flower
B. Leaf
C. Stem
D. Root
![]() Q. 135 Antivenom injection contains preformed antibodies while polio drops that are administered into the body contain:
Q. 135 Antivenom injection contains preformed antibodies while polio drops that are administered into the body contain:
A. Activated pathogens
B. Harvested antibodies
C. Gamma globulin
D. Attenuated pathogens
![]() Q. 136 Which one of the following cell organelles is enclosed by a single membrane?
Q. 136 Which one of the following cell organelles is enclosed by a single membrane?
A. Mitochondria
B. Chloroplasts
C. Lysosomes
D. Nuclei
![]() Q. 137 Lack of relaxation between successive stimuli in sustained muscle contraction is known as:
Q. 137 Lack of relaxation between successive stimuli in sustained muscle contraction is known as:
A. Spasm
B. Fatigue
C. Tetanus
D. Tonus
![]() Q. 138 Which of the following is not a stem modification?
Q. 138 Which of the following is not a stem modification?
A. Pitcher of Nepenthes
B. Thorns of citrus
C. Tendrils of cucumber
D. Flattened structures of Opuntia
![]() Q. 139 Water soluble pigments found in plant cell vacuoles are:
Q. 139 Water soluble pigments found in plant cell vacuoles are:
A. Xanthophylls
B. Chlorophylls
C. Carotenoids
D. Anthocyanins
![]() Q. 140 Select the correct statement:
Q. 140 Select the correct statement:
A. Gymnosperms are both homosporous and heterosporous
B. Salvinia, Ginkgo and Pinus all are gymnosperms
C. Sequoia is one of the tallest trees
D. The leaves of gymnosperms are not well adapted to extremes of climate
![]() Q. 141 Which of the following is not required for any of the techniques of DNA fingerprinting available at present?
Q. 141 Which of the following is not required for any of the techniques of DNA fingerprinting available at present?
A. Polymerase chain reaction
B. Zinc finger analysis
C. Restriction enzymes
D. DNA-DNA hybridization
![]() Q. 142 Which type of tissue correctly matches with its location?
Q. 142 Which type of tissue correctly matches with its location?
A. Tissue – Smooth muscle Location – Wall of intestine
B. Tissue – Areolar tissue Location – Tendons
C. Tissue – Transitional epithelium Location – Tip of nose
D. Tissue – Cuboidal epithelium Location – Lining of stomach
![]() Q. 143 A plant in your garden avoids photorespiratory losses, has improved water use efficiency, shows high rates of photosynthesis at high temperatures and has improved efficiency of nitrogen utilisation. In which of the following physiological groups would you assign this plant?
Q. 143 A plant in your garden avoids photorespiratory losses, has improved water use efficiency, shows high rates of photosynthesis at high temperatures and has improved efficiency of nitrogen utilisation. In which of the following physiological groups would you assign this plant?
A. C₃
B. C₄
C. CAM
D. Nitrogen fixer
![]() Q. 144 Which of the following structures is homologous to the wing of a bird?
Q. 144 Which of the following structures is homologous to the wing of a bird?
A. Dorsal fin of a Shark
B. Wing of a Moth
C. Hind limb of Rabbit
D. Flipper of Whale
![]() Q. 145 Which of the following characteristic features always holds true for the corresponding group of animals?
Q. 145 Which of the following characteristic features always holds true for the corresponding group of animals?
A. Cartilaginous endoskeleton – Chondrichthyes
B. Viviparous – Mammalia
C. Possess a mouth with an upper and a lower jaw – Chordata
D. 3-chambered heart with one incompletely divided ventricle – Reptilia
![]() Q. 146 Which of the following statements is not true for cancer cells in relation to mutations?
Q. 146 Which of the following statements is not true for cancer cells in relation to mutations?
A. Mutations in proto-oncogenes accelerate the cell cycle.
B. Mutations destroy telomerase inhibitor.
C. Mutations inactivate the cell control.
D. Mutations inhibit production of telomerase.
![]() Q. 147 The amino acid Tryptophan is the precursor for the synthesis of :
Q. 147 The amino acid Tryptophan is the precursor for the synthesis of :
A. Melatonin and Serotonin
B. Thyroxine and Triiodothyronine
C. Estrogen and Progesterone
D. Cortisol and Cortisone
![]() Q. 148 Following are the two statements regarding the origin of life :
Q. 148 Following are the two statements regarding the origin of life :
(a) The earliest organisms that appeared on the earth were non-green and presumably anaerobes.
(b) The first autotrophic organisms were the chemoautotrophs that never released oxygen.
Of the above statements which one of the following options is correct?
A. (a) is correct but (b) is false.
B. (b) is correct but (a) is false.
C. Both (a) and (b) are correct.
D. Both (a) and (b) are false.
![]() Q. 149 Reduction in pH of blood will:
Q. 149 Reduction in pH of blood will:
A. reduce the rate of heart beat.
B. reduce the blood supply to the brain.
C. decrease the affinity of hemoglobin with oxygen.
D. release bicarbonate ions by the liver.
![]() Q. 150 Analogous structures are a result of:
Q. 150 Analogous structures are a result of:
A. Divergent evolution
B. Convergent evolution
C. Shared ancestry
D. Stabilizing selection
![]() Q. 151 Which of the following is a restriction endonuclease?
Q. 151 Which of the following is a restriction endonuclease?
A. Hind II
B. Protease
C. DNase I
D. RNase
![]() Q. 152 The term ecosystem was coined by:
Q. 152 The term ecosystem was coined by:
A. E.P’. Odum
B. A.G. Tansley
C. E. Haeckel
D. E. Warming
![]() Q. 153 Which one of the following statements is wrong?
Q. 153 Which one of the following statements is wrong?
A. Sucrose is a disaccharide.
B. Cellulose is a polysaccharide.
C. Uracil is a pyrimidine.
D. Glycine is a sulphur containing amino acid.
![]() Q. 154 In bryophytes and pteridophytes, transport of male gametes requires:
Q. 154 In bryophytes and pteridophytes, transport of male gametes requires:
A. Wind
B. Insects
C. Birds
D. Water
![]() Q. 155 When does the growth rate of a population following the logistic model equal zero? The logistic model is given as dN/dt = rN(1-N/K):
Q. 155 When does the growth rate of a population following the logistic model equal zero? The logistic model is given as dN/dt = rN(1-N/K):
A. when N/K is exactly one.
B. when N nears the carrying capacity of the habitat.
C. when N/K equals zero.
D. when death rate is greater than birth rate.
![]() Q. 156 Which one of the following statements is not true?
Q. 156 Which one of the following statements is not true?
A. Tapetum helps in the dehiscence of anther
B. Exine of pollen grains is made up of sporopollenin
C. Pollen grains of many species cause severe allergies
D. Stored pollen in liquid nitrogen can be used in the crop breeding programmes
![]() Q. 157 Which of the following would appear as the pioneer organisms on bare rocks?
Q. 157 Which of the following would appear as the pioneer organisms on bare rocks?
A. Lichens
B. Liverworts
C. Mosses
D. Green algae
![]() Q. 158 Which one of the following is the starter codon?
Q. 158 Which one of the following is the starter codon?
A. AUG
B. UGA
C. UAA
D. UAG
![]() Q. 159 Which one of the following characteristics is not shared by birds and mammals?
Q. 159 Which one of the following characteristics is not shared by birds and mammals?
A. Ossified endoskeleton
B. Breathing using lungs
C. Viviparity
D. Warm blooded nature
![]() Q. 160 Nomenclature is governed by certain universal rules. Which one of the following is contrary to the rules of nomenclature?
Q. 160 Nomenclature is governed by certain universal rules. Which one of the following is contrary to the rules of nomenclature?
A. Biological names can be written in any language
B. The first word in a biological name represents the genus name, and the second is a specific epithet
C. The names are written in Latin and are italicised
D. When written by hand, the names are to be underlined
![]() Q. 161 Blood pressure in the pulmonary artery is:
Q. 161 Blood pressure in the pulmonary artery is:
A. same as that in the aorta.
B. more than that in the carotid.
C. more than that in the pulmonary vein.
D. less than that in the venae cavae.
![]() Q. 162 Cotyledon of maize grain is called:
Q. 162 Cotyledon of maize grain is called:
A. plumule
B. coleorhiza
C. coleoptile
D. scutellum
![]() Q. 163 In the stomach, gastric acid is secreted by the :
Q. 163 In the stomach, gastric acid is secreted by the :
A. gastrin secreting cells
B. parietal cells
C. peptic cells
D. acidic cells
![]() Q. 164 Depletion of which gas in the atmosphere can lead to an increased incidence of skin cancers:
Q. 164 Depletion of which gas in the atmosphere can lead to an increased incidence of skin cancers:
A. Nitrous oxide
B. Ozone
C. Ammonia
D. Methane
![]() Q. 165 Chrysophytes, Euglenoids, Dinoflagellates and Slime moulds are included in the kingdom:
Q. 165 Chrysophytes, Euglenoids, Dinoflagellates and Slime moulds are included in the kingdom:
A. Monera
B. Protista
C. Fungi
D. Animalia
![]() Q. 166 Water vapour comes out from the plant leaf through the stomatal opening. Through the same stomatal opening carbon dioxide diffuses into the plant during photosynthesis. Reason out the above statements using one of following options:
Q. 166 Water vapour comes out from the plant leaf through the stomatal opening. Through the same stomatal opening carbon dioxide diffuses into the plant during photosynthesis. Reason out the above statements using one of following options:
A. Both processes cannot happen simultaneously.
B. Both processes can happen together because the diffusion coefficient of water and CO₂ is different.
C. The above processes happen only during night time.
D. One process occurs during day time, and the other at night.
![]() Q. 167 In mammals, which blood vessel would normally carry largest amount of urea?
Q. 167 In mammals, which blood vessel would normally carry largest amount of urea?
A. Renal Vein
B. Dorsal Aorta
C. Hepatic Vein
D. Hepatic Portal Vein
![]() Q. 168 Seed formation without fertilization in flowering plants involves the process of:
Q. 168 Seed formation without fertilization in flowering plants involves the process of:
A. Sporulation
B. Budding
C. Somatic hybridization
D. Apomixis
![]() Q. 169 Which of the following is wrongly matched in the given table?
Q. 169 Which of the following is wrongly matched in the given table?
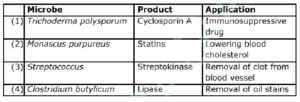
A. 1
B. 2
C. 3
D. 4
![]() Q. 170 In a testcross involving F₁ dihybrid flies, more parental-type offspring were produced than the recombinant-type offspring. This indicates:
Q. 170 In a testcross involving F₁ dihybrid flies, more parental-type offspring were produced than the recombinant-type offspring. This indicates:
A. The two genes are located on two different chromosomes.
B. Chromosomes failed to separate during meiosis.
C. The two genes are linked and present on the same chromosome.
D. Both of the characters are controlled by more than one gene.
![]() Q. 171 It is much easier for a small animal to run uphill than for a large animal, because:
Q. 171 It is much easier for a small animal to run uphill than for a large animal, because:
A. It is easier to carry a small body weight
B. Smaller animals have a higher metabolic rate
C. Small animals have a lower O₂ requirement
D. The efficiency of muscles in large animals is less than in the small animals.
![]() Q. 172 Which of the following is not a characteristic feature during mitosis in somatic cells?
Q. 172 Which of the following is not a characteristic feature during mitosis in somatic cells?
A. Spindle fibres
B. Disappearance of nucleolus
C. Chromosome movement
D. Synapsis
![]() Q. 173 Which of the following statements is not correct?
Q. 173 Which of the following statements is not correct?
A. pollen grains of many species can germinate on the stigma of a flower, but only one pollen tube of the same species grows into the style.
B. Insects that consume pollen or nectar without bringing about pollination are called pollen/nectar robbers.
C. Pollen germination and pollen tube growth are regulated by chemical components of pollen interacting with those of the pistil.
D. Some reptiles have also been reported as pollinators in some plant species.
![]() Q. 174 Specilised epidermal cells surrounding the guard cells are called:
Q. 174 Specilised epidermal cells surrounding the guard cells are called:
A. Complementary cells
B. Subsidiary cells
C. Bulliform cells
D. Lenticels
![]() Q. 175 Which of the following guards the opning of hepatopancreatic duct into the duodenum?
Q. 175 Which of the following guards the opning of hepatopancreatic duct into the duodenum?
A. Semilunar valve
B. Ileocaecal valve
C. Pyloric sphincter
D. Sphincter of Oddi
![]() Q. 176 Stems modified into flat green organs performing the functions of leaves are known as:
Q. 176 Stems modified into flat green organs performing the functions of leaves are known as:
A. Cladodes
B. Phyllodes
C. Phylloclades
D. Scales
![]() Q. 177 The primitive prokaryotes responsible for the production of biogas from the dung of ruminant animals, include the:
Q. 177 The primitive prokaryotes responsible for the production of biogas from the dung of ruminant animals, include the:
A. Halophiles
B. Thermoacidophiles
C. Methanogens
D. Eubacteria
![]() Q. 178 A river with an inflow of domestic sewage rich in organic waste may result in:
Q. 178 A river with an inflow of domestic sewage rich in organic waste may result in:
A. Drying of the river very soon due to algal bloom.
B. Increased population of aquatic food web organisms.
C. An increased production of fish due to biodegradable nutrients.
D. Death of fish due to lack of oxygen.
![]() Q. 179 A cell at telophase stage is observed by a student in a plant brought from the field. He tells his teacher that this cell is not like other cells at telophase stage. There is no formation of cell plate and thus the cell is containing more number of chromosomes as compared to other dividing cells. This would result in:
Q. 179 A cell at telophase stage is observed by a student in a plant brought from the field. He tells his teacher that this cell is not like other cells at telophase stage. There is no formation of cell plate and thus the cell is containing more number of chromosomes as compared to other dividing cells. This would result in:
A. Aneuploidy
B. Polyploidy
C. Somaclonal variation
D. Polyteny
![]() Q. 180 A typical fat molecule is made up of:
Q. 180 A typical fat molecule is made up of:
A. Three glycerol molecules and one fatty acid molecule
B. One glycerol and three fatty acid molecules
C. One glycerol and one fatty acid molecule
D. Three glycerol and three fatty acid molecules
| Question | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Answer | B | A | A | C | D | D | A | D | C | B |
| Question | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Answer | C | C | B | A | D | D | A | D | A | A |
| Question | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Answer | B | B | A | A | B | C | C | D | D | B |
| Question | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 |
| Answer | D | D | D | D | C | B | B | C | D | A |
| Question | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 |
| Answer | C | D | B | A | B | B | A | B | A | B |
| Question | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 |
| Answer | C | C | A | D | B | C | A | A | D | D |
| Question | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 |
| Answer | D | A | A | C | B | B | A | A | B | A |
| Question | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 |
| Answer | A | D | A | C | AA | D | A | B | A | A |
| Question | 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 | 89 | 90 |
| Answer | D | A | A | B | B | A | B | A | D | D |
| Question | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 |
| Answer | C | D | D | D | C | B | B | D | A | A |
| Question | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | 110 |
| Answer | C | D | B | B | B | B | C | D | B | A |
| Question | 111 | 112 | 113 | 114 | 115 | 116 | 117 | 118 | 119 | 120 |
| Answer | B | B | C | C | A | A | C | A | A | C |
| Question | 121 | 122 | 123 | 124 | 125 | 126 | 127 | 128 | 129 | 130 |
| Answer | B | A | D | B | C | B | C | D | C | D |
| Question | 131 | 132 | 133 | 134 | 135 | 136 | 137 | 138 | 139 | 140 |
| Answer | D | A | B | D | D | C | C | A | D | C |
| Question | 141 | 142 | 143 | 144 | 145 | 146 | 147 | 148 | 149 | 150 |
| Answer | B | A | B | D | A | D | A | C | C | B |
| Question | 151 | 152 | 153 | 154 | 155 | 156 | 157 | 158 | 159 | 160 |
| Answer | A | B | D | D | A | A | A | A | C | A |
| Question | 161 | 162 | 163 | 164 | 165 | 166 | 167 | 168 | 169 | 170 |
| Answer | C | D | B | B | B | B | C | D | D | C |
| Question | 171 | 172 | 173 | 174 | 175 | 176 | 177 | 178 | 179 | 180 |
| Answer | B | D | A | B | D | C | C | D | B | B |

