![]() NEET 2016 Phase-II
NEET 2016 Phase-II
|
Section |
Questions |
Marks |
|
Physics |
45 Questions (1 – 45) |
180 |
|
Chemistry |
45 Questions (46 – 90) |
180 |
|
Biology |
90 Questions (91 – 180) |
360 |
![]() Q. 1 Planck’s constant (h), speed of light in vacuum (c) and Newton’s gravitational constant (G) are three fundamental constants. Which of the following combinations of these has the dimension of length?
Q. 1 Planck’s constant (h), speed of light in vacuum (c) and Newton’s gravitational constant (G) are three fundamental constants. Which of the following combinations of these has the dimension of length?
A. √(hG)/c^(3/2)
B. √hG/c^(5/2)
C. √(hc/G)
D. √Gc/h^(3/2)
![]() Q. 2 Two cars P and Q start from a point at the same time in a straight line and their positions are represented by xP(t) = at + bt² and xQ(t) = ft – t². At what time do the cars have the same velocity?
Q. 2 Two cars P and Q start from a point at the same time in a straight line and their positions are represented by xP(t) = at + bt² and xQ(t) = ft – t². At what time do the cars have the same velocity?
A. (a – f)/(1 + b)
B. (a + f)/(2b – 1)
C. (a + f)/(21 + b)
D. (f – a)/(2 + 2b)
![]() Q. 3 In the given figure, a = 15 m/s² represents the total acceleration of a particle moving in the clockwise direction in a circle of radius R = 2.5 m at a given instant of time. The speed of the particle is
Q. 3 In the given figure, a = 15 m/s² represents the total acceleration of a particle moving in the clockwise direction in a circle of radius R = 2.5 m at a given instant of time. The speed of the particle is
A. 4.5 m/s
B. 5.0 m/s
C. 5.7 m/s
D. 6.2 m/s
![]() Q. 4 A rigid ball of mass m strikes a rigid wall at 60⁰ and gets reflected without loss of speed as shown in the figure below. The value of impulse imparted by the wall on the ball will be
Q. 4 A rigid ball of mass m strikes a rigid wall at 60⁰ and gets reflected without loss of speed as shown in the figure below. The value of impulse imparted by the wall on the ball will be

A. mV
B. 2mV
C. mV/2
D. mV/3
![]() Q. 5 A bullet of mass 10 g moving horizontally with a velocity of 400 m/s strikes a wooden block of mass 2 kg which is suspended by a light inextensible string of length 5 m. As a result, the centre of gravity of the block is found to rise a vertical distance of 10 cm. The speed of the bullet after it emerges out horizontally from the block will be
Q. 5 A bullet of mass 10 g moving horizontally with a velocity of 400 m/s strikes a wooden block of mass 2 kg which is suspended by a light inextensible string of length 5 m. As a result, the centre of gravity of the block is found to rise a vertical distance of 10 cm. The speed of the bullet after it emerges out horizontally from the block will be
A. 100 m/s
B. 80 m/s
C. 120 m/s
D. 160 m/s
![]() Q. 6 Two identical balls A and B having velocities of 0.5 m/s and -0.3 m/s, respectively collide elastically in one dimension. The velocities of B and A after the collision respectively will be
Q. 6 Two identical balls A and B having velocities of 0.5 m/s and -0.3 m/s, respectively collide elastically in one dimension. The velocities of B and A after the collision respectively will be
A. -0.5 m/s and 0.3 m/s
B. 0.5 m/s and -0.3 m/s
C. -0.3 m/s and 0.5 m/s
D. 0.3 m/s and 0.5 m/s
![]() Q. 7 A particle moves from a point -2iˆ + 5jˆ to 4jˆ + 3kˆ when a force of 4iˆ + 3jˆ N is applied. How much work has been done by the force?
Q. 7 A particle moves from a point -2iˆ + 5jˆ to 4jˆ + 3kˆ when a force of 4iˆ + 3jˆ N is applied. How much work has been done by the force?
A. 8 J
B. 11 J
C. 5 J
D. 2 J
![]() Q. 8 Two rotating bodies a and ᵦ of masses m and 2m with moments of inertia Iₐ and Iᵦ (Iᵦ > Iₐ) have equal kinetic energy of rotation. If Lₐ and Lᵦ be their angular momenta respectively, then
Q. 8 Two rotating bodies a and ᵦ of masses m and 2m with moments of inertia Iₐ and Iᵦ (Iᵦ > Iₐ) have equal kinetic energy of rotation. If Lₐ and Lᵦ be their angular momenta respectively, then
A. Lₐ = Lᵦ/2
B. Lₐ = 2Lᵦ
C. Lᵦ > Lₐ
D. Lₐ > Lᵦ
![]() Q. 9 A solid sphere of mass m and radius R is rotating about its diameter. A solid cylinder of the same mass and same radius is also rotating about its geometrical axis with an angular speed twice that of the sphere. The ratio of their kinetic energies of rotation (Esphere / Ecylinder) will be
Q. 9 A solid sphere of mass m and radius R is rotating about its diameter. A solid cylinder of the same mass and same radius is also rotating about its geometrical axis with an angular speed twice that of the sphere. The ratio of their kinetic energies of rotation (Esphere / Ecylinder) will be
A. 2:3
B. 1:5
C. 1:4
D. 3:1
![]() Q. 10 A light rod of length L has two masses m₁ and m₂ attached to its two ends. The moment of inertia of the system about an axis perpendicular to the rod and passing through the centre of mass is
Q. 10 A light rod of length L has two masses m₁ and m₂ attached to its two ends. The moment of inertia of the system about an axis perpendicular to the rod and passing through the centre of mass is
A. √m₁ √m₂ (L²)
B. m₁ m₂ (L²) / (m₁ + m₂)
C. (m₁ + m₂) / m ₁m₂ (L²)
D. (m₁ + m₂) L²
![]() Q. 11 Starting from the centre of the Earth having radius R, the variation of g (acceleration due to gravity) is shown by
Q. 11 Starting from the centre of the Earth having radius R, the variation of g (acceleration due to gravity) is shown by
A. (a)
B. (b)
C. (c)
D. (d)
![]() Q. 12 A satellite of mass m is orbiting the Earth (of radius R) at a height h from its surface. The total energy of the satellite in terms of g₀, the value of acceleration due to gravity at the Earth’s surface, is
Q. 12 A satellite of mass m is orbiting the Earth (of radius R) at a height h from its surface. The total energy of the satellite in terms of g₀, the value of acceleration due to gravity at the Earth’s surface, is
A. -2mg₀R² / (R+h)
B. mg₀R² / 2(R+h)
C. -mg₀R² / 2(R+h)
D. Rmg₀R² / (R+h)
![]() Q. 13 A rectangular film of liquid is extended from (4 cm x 2 cm) to (5 cm x 4 cm). If the work done is 3 x 10⁴ J, the value of the surface tension of the liquid is
Q. 13 A rectangular film of liquid is extended from (4 cm x 2 cm) to (5 cm x 4 cm). If the work done is 3 x 10⁴ J, the value of the surface tension of the liquid is
A. 0.250 Nm-1
B. 0.125 Nm-1
C. 0.2 Nm-1
D. 8.0 Nm-1
![]() Q. 14 Three liquids of densities ρ1, ρ2 and ρ3 (with ρ1 > ρ2 > ρ3), having the same value of surface tension T, rise to the same height in three identical capillaries. The angles of contact θ1, θ2 and θ3 obey
Q. 14 Three liquids of densities ρ1, ρ2 and ρ3 (with ρ1 > ρ2 > ρ3), having the same value of surface tension T, rise to the same height in three identical capillaries. The angles of contact θ1, θ2 and θ3 obey
A. 2^Π > θ1 > θ2 > θ3 ≥ 0
B. 0 ≤ θ1 < θ2 < θ3 < 2^Π
C. 2^Π < θ1 < θ2 < θ3 < Π
D. Π > θ1 > θ2 > θ3 > 2^Π
![]() Q. 15 Two identical bodies are made of a material for which the heat capacity increases with temperature. One of these is at 100 °C, while the other one is at 0 °C. If the two bodies are brought into contact, then, assuming no heat loss, the final common temperature is
Q. 15 Two identical bodies are made of a material for which the heat capacity increases with temperature. One of these is at 100 °C, while the other one is at 0 °C. If the two bodies are brought into contact, then, assuming no heat loss, the final common temperature is
A. 50 °C
B. More than 50 °C
C. Less than 50 °C but greater than 0 °C
D. 0 °C
![]() Q. 16 A body cools from a temperature 3T to 2T in 10 minutes. The room temperature is T. Assume that Newton’s law of cooling is applicable. The temperature of the body at the end of next of 10 minutes will be
Q. 16 A body cools from a temperature 3T to 2T in 10 minutes. The room temperature is T. Assume that Newton’s law of cooling is applicable. The temperature of the body at the end of next of 10 minutes will be
A. 7/4 T
B. 3/2 T
C. 4/3 T
D. T
![]() Q. 17 One mole of an ideal monatomic gas undergoes a process described by the equation PV³ = constant. The heat capacity of the gas during this process is
Q. 17 One mole of an ideal monatomic gas undergoes a process described by the equation PV³ = constant. The heat capacity of the gas during this process is
A. (3/2) R
B. (5/2) R
C. 2R
D. R
![]() Q. 18 The temperature inside a refrigerator is t₂ °C and the room temperature is t₁ °C. The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be
Q. 18 The temperature inside a refrigerator is t₂ °C and the room temperature is t₁ °C. The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be
A. t₁/ (t₁ – t₂)
B. (t₁ + 273) / (t₁ – t₂)
C. (t₂ + 273) / (t₁ – t₂)
D. (t₁ + t₂) / (t₁ + 273)
![]() Q. 19 A given sample of an ideal gas occupies a volume V at a pressure P and absolute temperature T. The mass of each molecule of the gas is m. Which of the following gives the density of the gas?
Q. 19 A given sample of an ideal gas occupies a volume V at a pressure P and absolute temperature T. The mass of each molecule of the gas is m. Which of the following gives the density of the gas?
A. P/(kT)
B. Pm/(kT)
C. P/(kTV)
D. mkT
![]() Q. 20 A body of mass m is attached to the lower end of a spring whose upper end is fixed. The spring has negligible mass. When the mass m is slightly pulled down and released, it oscillates with a time period of 3 s. When the mass m is increased by 1 kg, the time period of oscillations becomes 5 s. The value of m in kg is :
Q. 20 A body of mass m is attached to the lower end of a spring whose upper end is fixed. The spring has negligible mass. When the mass m is slightly pulled down and released, it oscillates with a time period of 3 s. When the mass m is increased by 1 kg, the time period of oscillations becomes 5 s. The value of m in kg is :
A. 3/4
B. 4/3
C. 16/9
D. 9/16
![]() Q. 21 The second overtone of an open organ pipe has the same frequency as the first overtone of a closed pipe L meter long. The length of the open pipe will be
Q. 21 The second overtone of an open organ pipe has the same frequency as the first overtone of a closed pipe L meter long. The length of the open pipe will be
A. L
B. 2L
C. L/2
D. 4L
![]() Q. 22 Three sound waves of equal amplitudes have frequencies (n – 1), n, (n + 1). They superimpose to give beats. The number of beats produced per second will be
Q. 22 Three sound waves of equal amplitudes have frequencies (n – 1), n, (n + 1). They superimpose to give beats. The number of beats produced per second will be
A. 1
B. 4
C. 3
D. 2
![]() Q. 23 An electric dipole is placed at an angle of 30° with an electric field intensity 2 × 10^5 N/C. It experiences a torque equal to 4 Nm. The charge on the dipole, if the dipole length is 2 cm, is
Q. 23 An electric dipole is placed at an angle of 30° with an electric field intensity 2 × 10^5 N/C. It experiences a torque equal to 4 Nm. The charge on the dipole, if the dipole length is 2 cm, is
A. 8 mC
B. 2 mC
C. 5 mC
D. 7 μC
![]() Q. 24 A parallel- plate capacitor of area A, plate separation d and capacitance C is filled with four dielectric materials having dielectric constant k1, k₂, k₃, and k₄ as shown in the figure below. If a single dielectric material is to be used to have the same capacitance C in this capacitor, then its dielectric constant k is given by
Q. 24 A parallel- plate capacitor of area A, plate separation d and capacitance C is filled with four dielectric materials having dielectric constant k1, k₂, k₃, and k₄ as shown in the figure below. If a single dielectric material is to be used to have the same capacitance C in this capacitor, then its dielectric constant k is given by
A. k = k₁ + k₂ + k₃ + 3 (k₄)
B. k = (2/3)(k₁ + k₂ + k₃) + 2 (k₄ )
C. 2/k = 3/(k₁ + k₂ + k₃) + 1/k₄
D. 1/k = 1/k₁ + 1/k₂ + 1/k₃ + 3/2 (k₄ )
![]() Q. 25 The potential difference (VA – VB) between the points A and B in the given figure is
Q. 25 The potential difference (VA – VB) between the points A and B in the given figure is
A. – 3 V
B. + 3 V
C. + 6 V
D. + 9 V
![]() Q. 26 A filament bulb (500 W, 100 V) is to be used in a 230 V main supply. When a resistance R is connected in series, it works perfectly and the bulb consumes 500 W. The value of R is
Q. 26 A filament bulb (500 W, 100 V) is to be used in a 230 V main supply. When a resistance R is connected in series, it works perfectly and the bulb consumes 500 W. The value of R is
A. 230 Ω
B. 46 Ω
C. 26 Ω
D. 13 Ω
![]() Q. 27 A long wire carrying a steady current is bent into a circular loop of one turn. The magnetic field at the centre of the loop is B. It is then bent into a circular coil of n turns. The magnetic field at the centre of this coil of n turns will be
Q. 27 A long wire carrying a steady current is bent into a circular loop of one turn. The magnetic field at the centre of the loop is B. It is then bent into a circular coil of n turns. The magnetic field at the centre of this coil of n turns will be
A. nB
B. n²B
C. 2nB
D. 2n²B
![]() Q. 28 A bar magnet is hung by a thin cotton thread in a uniform horizontal magnetic field and is in equilibrium state. The energy required to rotate it by 60° is W. Now the torque required to keep the magnet in this new position is
Q. 28 A bar magnet is hung by a thin cotton thread in a uniform horizontal magnetic field and is in equilibrium state. The energy required to rotate it by 60° is W. Now the torque required to keep the magnet in this new position is
A. W/√3
B. √3 W
C. √3/2 W
D. 2W / √3
![]() Q. 29 An electron is moving in a circular path under the influence of a transverse magnetic field of 3.57 × 10^(–2) T. If the value of e/m is 1.76 × 10^(11) C/kg, the frequency of revolution of the electron is
Q. 29 An electron is moving in a circular path under the influence of a transverse magnetic field of 3.57 × 10^(–2) T. If the value of e/m is 1.76 × 10^(11) C/kg, the frequency of revolution of the electron is
A. 1 GHz
B. 100 MHz
C. 62.8 MHz
D. 6.28 MHz
![]() Q. 30 Which of the following combinations should be selected for better tuning of an L-C-R circuit used for communication?
Q. 30 Which of the following combinations should be selected for better tuning of an L-C-R circuit used for communication?
A. R = 20 Ω, L = 1.5 H, C = 35μF
B. R = 25 Ω, L = 2.5 H, C = 45μF
C. R = 15 Ω, L = 3.5 H, C = 30μF
D.R = 25 Ω, L = 1.5 H, C = 45μF
![]() Q. 31 A uniform magnetic field is restricted within a region of radius r. The magnetic field changes with time at a rate dB/dt . Loop 1 of radius R > r encloses the region r and loop 2 of radius R is outside the region of magnetic field as shown in the figure below. Then the e.m.f. generated is
Q. 31 A uniform magnetic field is restricted within a region of radius r. The magnetic field changes with time at a rate dB/dt . Loop 1 of radius R > r encloses the region r and loop 2 of radius R is outside the region of magnetic field as shown in the figure below. Then the e.m.f. generated is
A. Zero in loop 1 and zero in loop 2
B. -dB/dt ( πr² ) in loop 1 and -dB/dt ( πr² ) in loop 2
C. -dB/dt ( πr² ) in loop 1 and zero in loop 2
D. -dB/dt ( πR² ) in loop 1 and zero in loop 2
![]() Q. 32 The potential differences across the resistance, capacitance and inductance are 80 V, 40 V and 100 V respectively in an L-C-R circuit. The power factor of this circuit is
Q. 32 The potential differences across the resistance, capacitance and inductance are 80 V, 40 V and 100 V respectively in an L-C-R circuit. The power factor of this circuit is
A. 0.4
B. 0.5
C. 0.8
D. 1.0
![]() Q. 33 A 100 Ω resistance and a capacitor of 100 Ω reactance are connected in series across a 220 V source. When the capacitor is 50% charged, the peak value of the displacement current is
Q. 33 A 100 Ω resistance and a capacitor of 100 Ω reactance are connected in series across a 220 V source. When the capacitor is 50% charged, the peak value of the displacement current is
A. 2.2 A
B. 11 A
C. 4.4 A
D. 11 √2 A
![]() Q. 34 Two identical glass (μg = 3/2) equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination is
Q. 34 Two identical glass (μg = 3/2) equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination is
A. f/3
B. f
C. 4f/3
D. 3f/4
![]() Q. 35 An air bubble in a glass slab with refractive index 1.5 (near normal incidence) is 5 cm deep when viewed from one surface and 3 cm deep when viewed from the opposite face. The thickness (in cm) of the slab is
Q. 35 An air bubble in a glass slab with refractive index 1.5 (near normal incidence) is 5 cm deep when viewed from one surface and 3 cm deep when viewed from the opposite face. The thickness (in cm) of the slab is
A. 8
B. 10
C. 12
D. 16
![]() Q. 36 The interference pattern is obtained with two coherent light sources of intensity ratio n. In the interference pattern, the ratio (Imax – Imin)/ (Imax + Imin), will be
Q. 36 The interference pattern is obtained with two coherent light sources of intensity ratio n. In the interference pattern, the ratio (Imax – Imin)/ (Imax + Imin), will be
A. √n/(n + 1)
B. √n/(n + 1)
C. √n/(n + 1)²
D. 2√n/(n + 1)²
![]() Q. 37 A person can see clearly objects only when they lie between 50 cm and 400 cm from his eyes. In order to increase the maximum distance of distinct vision to infinity, the type and power of the correcting lens, the person has to use, will be
Q. 37 A person can see clearly objects only when they lie between 50 cm and 400 cm from his eyes. In order to increase the maximum distance of distinct vision to infinity, the type and power of the correcting lens, the person has to use, will be
A. Convex, + 2.25 diopter
B. Concave, – 0.25 diopter
C. Concave, – 0.2 diopter
D. Convex, + 0.15 diopter
![]() Q. 38 A linear aperture whose width is 0.02 cm is placed immediately in front of a lens of focal length 60 cm. The aperture is illuminated normally by a parallel beam of wavelength 5 × 10^(-5) cm. The distance of the first dark band of the diffraction pattern from the centre of the screen is
Q. 38 A linear aperture whose width is 0.02 cm is placed immediately in front of a lens of focal length 60 cm. The aperture is illuminated normally by a parallel beam of wavelength 5 × 10^(-5) cm. The distance of the first dark band of the diffraction pattern from the centre of the screen is
A. 0.10 cm
B. 0.25 cm
C. 0.20 cm
D. 0.15 cm
![]() Q. 39 Electrons of mass m with de-Broglie wavelength λ fall on the target in an X-ray tube. The cutoff wavelength (λ0) of the emitted X-ray is :-
Q. 39 Electrons of mass m with de-Broglie wavelength λ fall on the target in an X-ray tube. The cutoff wavelength (λ0) of the emitted X-ray is :-
A. λ0 = 2mcλ2/h
B. λ0 = 2h/mc
C. λ0 = 2m²c²λ³/h²
D. λ0 = λ
![]() Q. 40 Photons with energy 5 eV are incident on a cathode C in a photoelectric cell. The maximum energy of emitted photoelectrons is 2 eV. When photons of energy 6 eV are incident on C, no photoelectrons will reach the anode A, if the stopping potential of A relative to C is:-
Q. 40 Photons with energy 5 eV are incident on a cathode C in a photoelectric cell. The maximum energy of emitted photoelectrons is 2 eV. When photons of energy 6 eV are incident on C, no photoelectrons will reach the anode A, if the stopping potential of A relative to C is:-
A. +3 V
B. +4 V
C. -1 V
D. 3 V
![]() Q. 41 If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd orbit, it emits a photon of wavelength λ . When it jumps from the 4th orbit to the 3rd orbit, the corresponding wavelength of the photon will be :-
Q. 41 If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd orbit, it emits a photon of wavelength λ . When it jumps from the 4th orbit to the 3rd orbit, the corresponding wavelength of the photon will be :-
A. (16/25) λ
B. (9/16)/λ
C. (20/7) λ
D. (20/13) λ
![]() Q. 42 The half-life of a radioactive substance is 30 minutes. The time (in minutes) taken between 40% decay and 85% decay of the same radioactive substance is :-
Q. 42 The half-life of a radioactive substance is 30 minutes. The time (in minutes) taken between 40% decay and 85% decay of the same radioactive substance is :-
A. 15
B. 30
C. 45
D. 60
![]() Q. 43 For CE transistor amplifier, the audio signal voltage across the collector resistance of 2kΩ is 4 V. If the current amplification factor of the transistor is 100 and the base resistance is 1 kΩ, then the input signal voltage is :-
Q. 43 For CE transistor amplifier, the audio signal voltage across the collector resistance of 2kΩ is 4 V. If the current amplification factor of the transistor is 100 and the base resistance is 1 kΩ, then the input signal voltage is :-
A. 10 mV
B. 20 mV
C. 30 mV
D. 15 mV
![]() Q. 44 The given circuit has two ideal diodes connected as shown in the figure below. The current flowing through the resistance R1 will be
Q. 44 The given circuit has two ideal diodes connected as shown in the figure below. The current flowing through the resistance R1 will be
A. 2.5 A
B. 10.0 A
C. 1.43 A
D. 3.13 A
![]() Q. 45 What is the output Y in the following circuit, when all the three inputs A, B, C are first 0 and then 1?
Q. 45 What is the output Y in the following circuit, when all the three inputs A, B, C are first 0 and then 1?
A. 0, 1
B. 0, 0
C. 1, 0
D. 1, 1
![]() Q. 46 Which one of the following compounds shows the presence of intramolecular hydrogen bond?
Q. 46 Which one of the following compounds shows the presence of intramolecular hydrogen bond?
A. H2O2
B. HCN
C. Cellulose
D. Concentrated acetic acid
![]() Q. 47 The molar conductivity of a 0.5 mol/dm³ solution of AgNO3 with electrolytic conductivity of 5.76 × 10^(-3) S cm–1 at 298 K is
Q. 47 The molar conductivity of a 0.5 mol/dm³ solution of AgNO3 with electrolytic conductivity of 5.76 × 10^(-3) S cm–1 at 298 K is
A. 2.88 S cm²/mol
B. 11.52 S cm²/mol
C. 0.086 S cm²/mol
D. 28.8 S cm²/mol
![]() Q. 48 The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
Q. 48 The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
A. rate is proportional to the surface coverage
B. rate is inversely proportional to the surface coverage
C. rate is independent of the surface coverage
D. rate of decomposition is very slow
![]() Q. 49 The coagulation values in millimoles per litre of the electrolytes used for the coagulation of As₂S₃ are given below:
Q. 49 The coagulation values in millimoles per litre of the electrolytes used for the coagulation of As₂S₃ are given below:
I. (NaCl) = 52,
II. (BaCl₂) = 0.69,
III. (MgSO₄) = 0.22
The correct order of their coagulating power is
A. I > II > III
B. II > I > III
C. III > II > I
D. III > I > II
![]() Q. 50 During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is
Q. 50 During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is
A. 55 minutes
B. 110 minutes
C. 220 minutes
D. 330 minutes
![]() Q. 51 How many electrons can fit in the orbital for which n = 3 and l = 1?
Q. 51 How many electrons can fit in the orbital for which n = 3 and l = 1?
A. 2
B. 6
C. 10
D. 14
![]() Q. 52 For a sample of perfect gas when its pressure is changed isothermally from pᵢ to p, the entropy change is given by
Q. 52 For a sample of perfect gas when its pressure is changed isothermally from pᵢ to p, the entropy change is given by
A. Δ S =nR ln ( p / pᵢ )
B. Δ S =nR ln ( pᵢ / p )
C. Δ S =nRT ln ( p / pᵢ )
D. Δ S =RT ln ( pᵢ / p )
![]() Q. 53 The van’t Hoff factor (i) for a dilute aqueous solution of the strong electrolyte barium hydroxide is
Q. 53 The van’t Hoff factor (i) for a dilute aqueous solution of the strong electrolyte barium hydroxide is
A. 0
B. 1
C. 2
D. 3
![]() Q. 54 The percentage of pyridine (C₅H₅N) that forms pyridinium ion (C₅H₅N⁺H) in a 0.10 M aqueous pyridine solution (Kᵦ for C₅H₅N = 1.7 × 10⁻⁹) is
Q. 54 The percentage of pyridine (C₅H₅N) that forms pyridinium ion (C₅H₅N⁺H) in a 0.10 M aqueous pyridine solution (Kᵦ for C₅H₅N = 1.7 × 10⁻⁹) is
A. 0.0060%
B. 0.013%
C. 0.77%
D. 1.6%
![]() Q. 55 In calcium fluoride, having the fluorite structure, the coordination numbers for calcium ion Ca²⁺ and fluoride ion F⁻ are
Q. 55 In calcium fluoride, having the fluorite structure, the coordination numbers for calcium ion Ca²⁺ and fluoride ion F⁻ are
A. 4 and 2
B. 6 and 6
C. 8 and 4
D. 4 and 8
![]() Q. 56 If the E°cell for a given reaction has a negative value which of the following gives the correct relationships for the values of G° and Keq?
Q. 56 If the E°cell for a given reaction has a negative value which of the following gives the correct relationships for the values of G° and Keq?
A. ΔG° > 0; Keq < 1
B. ΔG° > 0; Keq > 1
C. ΔG° < 0; Keq > 1
D. ΔG° < 0; Keq < 1
![]() Q. 57 Which one of the following is incorrect for ideal solution?
Q. 57 Which one of the following is incorrect for ideal solution?
A. ΔHmix = 0
B. ΔUmix = 0
C. ΔP = Pobs – P(calculated by Raoult’s law) = 0
D. ΔGmix = 0
![]() Q. 58 The solubility of AgCl(s) with solubility product 1.6 × 10^(–10) in 0.1 M NaCl solution would be
Q. 58 The solubility of AgCl(s) with solubility product 1.6 × 10^(–10) in 0.1 M NaCl solution would be
A. 1.25 × 10^(-5) M
B. 1.6 × 10^(-9) M
C. 1.6 × 10^(-11) M
D. zero
![]() Q. 59 Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are
Q. 59 Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are
A. 40, 30
B. 60, 40
C. 20, 30
D. 30, 20
![]() Q. 60 The number of electrons delivered at the cathode during electrolysis by a current of 1 ampere in 60 seconds is (charge on electron = 1.60 ×10^(–19) C)
Q. 60 The number of electrons delivered at the cathode during electrolysis by a current of 1 ampere in 60 seconds is (charge on electron = 1.60 ×10^(–19) C)
A. 6 × 10^(23)
B. 6 × 10^(20)
C. 3.75 × 10^(20)
D. 7.48 × 10^(23)
![]() Q. 61 Boric acid is an acid because its molecule
Q. 61 Boric acid is an acid because its molecule
A. contains replaceable H+ ion
B. gives up a proton
C. accepts OH– from water releasing proton
D. combines with proton from water molecule
![]() Q. 62 AlF₃ is soluble in HF only in presence of KF. It is due to the formation of
Q. 62 AlF₃ is soluble in HF only in presence of KF. It is due to the formation of
A. K₃ [ AlF₃H₃ ]
B. K₃ [ AlF₆ ]
C. AlH₃
D. K [ AlF₃H ]
![]() Q. 63 Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
Q. 63 Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
A. zinc is lighter than iron
B. zinc has lower melting point than iron
C. zinc has lower negative electrode potential than iron
D. zinc has higher negative electrode potential than iron
![]() Q. 64 The suspension of slaked lime in water is known as
Q. 64 The suspension of slaked lime in water is known as
A. Lime water
B. Quicklime
C. Milk of lime
D. Aqueous solution of slaked lime
![]() Q. 65 The hybridizations of atomic orbitals of nitrogen in NO2+, NO3- and NH4+ respectively are
Q. 65 The hybridizations of atomic orbitals of nitrogen in NO2+, NO3- and NH4+ respectively are
A. sp, sp³ and sp²
B. sp², sp³ and sp
C. sp, sp² and sp³
D. sp², sp and sp³
![]() Q. 66 Which of the following fluoro-compounds is most likely to behave as a Lewis base?
Q. 66 Which of the following fluoro-compounds is most likely to behave as a Lewis base?
A. BF3
B. PF3
C. CF4
D. SiF4
![]() Q. 67 Which of the following pairs of ions is isoelectronic and isostructural?
Q. 67 Which of the following pairs of ions is isoelectronic and isostructural?
A. (CO₃)²⁻, (NO₃)⁻
B. (ClO₃)⁻, (CO₃)²⁻
C. (SO₃)²⁻, (NO₃)⁻
D. (ClO₃)⁻, (SO₃)²⁻
![]() Q. 68 In context with beryllium, which one of the following statements is incorrect?
Q. 68 In context with beryllium, which one of the following statements is incorrect?
A. It is rendered passive by nitric acid.
B. It forms Be2C.
C. Its salts rarely hydrolyze.
D. Its hydride is electron-deficient and polymeric.
![]() Q. 69 Hot concentrated sulphuric acid is a moderately strong oxidizing agent. Which of the following reactions does not show oxidizing behavior?
Q. 69 Hot concentrated sulphuric acid is a moderately strong oxidizing agent. Which of the following reactions does not show oxidizing behavior?

A. (a)
B. (b)
C. (c)
D. (d)
![]() Q. 70 Which of the following pairs of d-orbitals will have electron density along the axes?
Q. 70 Which of the following pairs of d-orbitals will have electron density along the axes?
A. dz², dxz
B. dxz, dyz
C. dz² , dx²-y²
D. dxy , dx²-y²
![]() Q. 71 The correct geometry and hybridization for XeF4 are :
Q. 71 The correct geometry and hybridization for XeF4 are :
A. Octahedral, sp3d2
B. Trigonal bipyramidal, sp3d
C. Planar triangle, sp3d3
D. Square planar, sp3d2
![]() Q. 72 Among the following which one is a wrong statement?
Q. 72 Among the following which one is a wrong statement?
A. PH5 and BiCl5 do not exist
B. pπ-dπ bonds are present in SO2
C. SeF4 and CH4 have same shape
D. I3+ has bent geometry
![]() Q. 73 The correct increasing order of trans-effect of the following species is :
Q. 73 The correct increasing order of trans-effect of the following species is :
A. (NH3) > (CN–) > (Br–) > (C6H5–)
B. (CN–) > (C6H5–) > (Br–) > (NH3)
C. (Br–) > (CN–) > (NH3) > (C6H5–)
D. (CN–) > (Br–) > (C6H5–) >(NH3)
![]() Q. 74 Which one of the followng statements related to lanthanons is incorrect?
Q. 74 Which one of the followng statements related to lanthanons is incorrect?
A. Europium shows +2 oxidation state.
B. The basicity decreases as the ionic radius decreases from Pr to Lu.
C. All the lanthanons are much more reactive than aluminium
D. Ce(+4) solutions are widely used as oxidizing agent in volumetric analysis
![]() Q. 75 Jahn-Teller effect not observed in high spin complexes of :-
Q. 75 Jahn-Teller effect not observed in high spin complexes of :-
A. d7
B. d8
C. d4
D. d9
![]() Q. 76 Which of the following can be used as halide component for Friedel-Crafts reaction?
Q. 76 Which of the following can be used as halide component for Friedel-Crafts reaction?
A. Chlorobenzene
B. Bromobenzene
C. Chloroethene
D. Isopropyl chloride
![]() Q. 77 In which of the following molecules, atoms are coplanar?
Q. 77 In which of the following molecules, atoms are coplanar?
A. (1)
B. (2)
C. (3)
D. (4)
![]() Q. 78 Which one of the following structures represents nylon 6,6 polymer?
Q. 78 Which one of the following structures represents nylon 6,6 polymer?
A. (1)
B. (2)
C. (3)
D. (4)
![]() Q. 79 In pyrrole, the electron density is maximum on
Q. 79 In pyrrole, the electron density is maximum on
A. 2 and 3
B. 3 and 4
C. 2 and 4
D. 2 and 5
![]() Q. 80 Which of the following compounds shall not produce propene by reaction with HBr followed by elimination or direct only elimination reaction?
Q. 80 Which of the following compounds shall not produce propene by reaction with HBr followed by elimination or direct only elimination reaction?
A. (1)
B. (2)
C. (3)
D. (4)
![]() Q. 81 Which one of the following nitro-compounds does not react with nitrous acid?
Q. 81 Which one of the following nitro-compounds does not react with nitrous acid?
A. (1)
B. (2)
C. (3)
D. (4)
![]() Q. 82 The central dogma of molecular genetics states that the genetic information flows from
Q. 82 The central dogma of molecular genetics states that the genetic information flows from
A. Amino acids → Proteins → DNA
B. DNA → Carbohydrates → Proteins
C. DNA → RNA → Proteins
D. DNA → RNA → Carbohydrates
![]() Q. 83 The correct corresponding order of names of following aldoses with configuration given below, respectively, is
Q. 83 The correct corresponding order of names of following aldoses with configuration given below, respectively, is
A. L-erythrose, L-threose, L-erythrose, D-threose
B. D-threose, D-erythrose, L-threose, L-erythrose
C. L-erythrose, L-threose, D-erythrose, D-threose
D. D-erythrose, D-threose, L-erythrose, L-threose
![]() Q. 84 In the given reaction, the product P is,
Q. 84 In the given reaction, the product P is,
A. (a)
B. (b)
C. (c)
D. (d)
![]() Q. 85 A given nitrogen containing aromatic compound aromatic A reacts with Sn/HCl, followed by HNO2 to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N 2O. The structure of the compound A is
Q. 85 A given nitrogen containing aromatic compound aromatic A reacts with Sn/HCl, followed by HNO2 to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N 2O. The structure of the compound A is
A. (a)
B. (b)
C. (c)
D. (d)
![]() Q. 86 Consider the reaction
Q. 86 Consider the reaction
CH3-CH2-CH2-Br + NaCN → CH3 –CH2 – CH2 –CN + NaBr
The reaction will be the fastest in
A. ethanol
B. methanol
C. N, N’ –dimethylformamide (DMF)
D. water
![]() Q. 87 The correct structure of the product A formed in the reaction
Q. 87 The correct structure of the product A formed in the reaction
A. (a)
B. (b)
C. (c)
D. (d)
![]() Q. 88 Which among the given molecules can exhibit tautomerism?
Q. 88 Which among the given molecules can exhibit tautomerism?
A. III only
B. Both I and III
C. Both I and II
D. Both II and III
![]() Q. 89 The correct order of the strengths of the carboxylic acids
Q. 89 The correct order of the strengths of the carboxylic acids
A. I> II >III
B. II > III > I
C. III > II > I
D. II > I > II
![]() Q. 90 The compound that will react most readily with gaseous bromine has the formula?
Q. 90 The compound that will react most readily with gaseous bromine has the formula?
A. C3H6
B. C2H2
C. C4H10
D. C2H4
![]() Q. 91 Which one of the following is wrong for fungi?
Q. 91 Which one of the following is wrong for fungi?
A. They are eukaryotic.
B. All fungi possess a purely cellulosic cell wall.
C. They are heterotrophic.
D. They are both unicellular and multicellular.
![]() Q. 92 Methanogens belong to
Q. 92 Methanogens belong to
A. Eubacteria
B. Archaebacteria
C. Dinoflagellates
D. Slime moulds
![]() Q. 93 Select the wrong statement
Q. 93 Select the wrong statement
A. The walls of diatoms are easily destructible.
B. ‘Diatomaceous earth’ is formed by the cell wall of diatoms.
C. Diatoms are chief producers in the ocean.
D. Diatoms are microscopic and float passively in water
![]() Q. 94 The label of a herbarium sheet does not carry information on
Q. 94 The label of a herbarium sheet does not carry information on
A. date of collection
B. name of collector
C. local names
D. height of the plant
![]() Q. 95 Conifers are adapted to tolerate extreme environmental conditions because of
Q. 95 Conifers are adapted to tolerate extreme environmental conditions because of
A. broad hardy leaves
B. superficial stomata
C. thick cuticle
D. presence of vessels
![]() Q. 96 Which one of the following statements is wrong?
Q. 96 Which one of the following statements is wrong?
A. Algae increase the level of dissolved oxygen in the immediate environment.
B. Algin is obtained from red algae, and carrageenan from brown algae.
C. Agar-agar is obtained from Gelidium and Gracilaria
D. Laminaria and Sargassum are used as food.
![]() Q. 97 The term ‘polyadelphous’ is related to
Q. 97 The term ‘polyadelphous’ is related to
A. gynoecium
B. androecium
C. corolla
D. calyx
![]() Q. 98 How many plants among Indigofera, Sesbania, Salvia, Allium, Aloe, mustard, groundnut, radish, gram and turnip have stamens with different lengths in their flowers?
Q. 98 How many plants among Indigofera, Sesbania, Salvia, Allium, Aloe, mustard, groundnut, radish, gram and turnip have stamens with different lengths in their flowers?
A. Three
B. Four
C. Five
D. Six
![]() Q. 99 Radial symmetry is found in the flowers of
Q. 99 Radial symmetry is found in the flowers of
A. Brassica
B. Trifolium
C. Pisum
D. Cassia
![]() Q. 100 Free-central placentation is found in
Q. 100 Free-central placentation is found in
A. Dianthus
B. Argemone
C. Brassica
D. Citrus
![]() Q. 101 Cortex is the region found between
Q. 101 Cortex is the region found between
A. epidermis and stele
B. pericycle and endodermis
C. endodermis and pith
D. endodermis and vascular bundle
![]() Q. 102 The balloon-shaped structures called tyloses
Q. 102 The balloon-shaped structures called tyloses
A. lysozyme
B. ribozyme
C. ligase
D. deoxyribonuclease
![]() Q. 103 A non-proteinaceous enzyme is
Q. 103 A non-proteinaceous enzyme is
A. Stroma
B. Lumen of thylakoids
C. Inter membrane space
D. Antennae complex
![]() Q. 104 Select the mismatch
Q. 104 Select the mismatch
A. Gas vacuoles – Green bacteria
B. Large central vacuoles – Animal cells
C. Protists – Eukaryotes
D. Methanogens – Prokaryotes
![]() Q. 105 Select the wrong statement.
Q. 105 Select the wrong statement.
A. Bacterial cell wall is made up of peptidoglycan.
B. Pili and fimbriae are mainly involved in motility of bacterial cells.
C. Cyanobacteria lack flagellated cells.
D. Mycoplasma is a wall-less microorganism.
![]() Q. 106 A cell organelle containing hydrolytic enzymes is
Q. 106 A cell organelle containing hydrolytic enzymes is
A. lysosome
B. microsome
C. ribosome
D. mesosome
![]() Q. 107 During cell growth, DNA synthesis takes place in
Q. 107 During cell growth, DNA synthesis takes place in
A. S Phase
B. G1 phase
C. G2 phase
D. M phase
![]() Q. 108 Which of the following biomolecules is common to respiration-mediated breakdown of fats, carbohydrates and proteins?
Q. 108 Which of the following biomolecules is common to respiration-mediated breakdown of fats, carbohydrates and proteins?
A. Glucose-6-phosphate
B. Fructose 1,6-bisphosphate
C. Pyruvic acid
D. Acetyl CoA
![]() Q. 109 A few drops of sap were collected by cutting across a plant stem by a suitable method. The sap was tested chemically. Which one of the following test results indicates that it is phloem sap?
Q. 109 A few drops of sap were collected by cutting across a plant stem by a suitable method. The sap was tested chemically. Which one of the following test results indicates that it is phloem sap?
A. Acidic
B. Alkaline
C. Low refractive index
D. Absence of sugar
![]() Q. 110 You are given a tissue with its potential for differentiation in an artificial culture. Which of the following pairs of hormones would you add to the medium to secure shoots as well as roots?
Q. 110 You are given a tissue with its potential for differentiation in an artificial culture. Which of the following pairs of hormones would you add to the medium to secure shoots as well as roots?
A. IAA and gibberellins
B. Auxin and cytokinin
C. Auxin and abscisic acid
D. Gibberellin and abscisic acid
![]() Q. 111 Phytochrome is a
Q. 111 Phytochrome is a
A. flavoprotein
B. glycoprotein
C. lipoprotein
D. chromoprotein
![]() Q. 112 Which is essential for the growth of root tip?
Q. 112 Which is essential for the growth of root tip?
A. Zn
B. Fe
C. Ca
D. Mn
![]() Q. 113 The process which makes major difference between C3 and C4 plants is
Q. 113 The process which makes major difference between C3 and C4 plants is
A. glycolysis
B. Calvin cycle
C. photorespiration
D. respiration
![]() Q. 114 Which one of the following statements is not correct?
Q. 114 Which one of the following statements is not correct?
A. Offspring produced by the asexual reproduction are called clone.
B. Microscopic, motile asexual reproductive structures are called zoospores.
C. In potato, banana and ginger, the plantlets arise from the internodes present in the modified stem.
D. Water hyacinth, growing in the standing water, drains oxygen from water that leads to the death of fishes.
![]() Q. 115 Which one of the following generates new genetic combinations leading to variation?
Q. 115 Which one of the following generates new genetic combinations leading to variation?
A. Vegetative reproduction
B. Parthenogenesis
C. Sexual reproduction
D. Nucellar polyembryony
![]() Q. 116 Match Column-I with Column-II and select the correct option using the codes given below:
Q. 116 Match Column-I with Column-II and select the correct option using the codes given below:
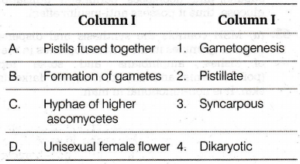
A. (1)
B. (2)
C. (3)
D. (4)
![]() Q. 117 In majority of angiosperms
Q. 117 In majority of angiosperms
A. egg has a filiform apparatus
B. there are numerous antipodal cells
C. reduction division occurs in the megaspore mother cells
D. a small central cell is present in the embryo sac
![]() Q. 118 Pollination in water hyacinth and water lily is brought about by the agency of
Q. 118 Pollination in water hyacinth and water lily is brought about by the agency of
A. water
B. insects or wind
C. birds
D. bats
![]() Q. 119 The ovule of an angiosperm is technically equivalent
Q. 119 The ovule of an angiosperm is technically equivalent
A. megasporangium
B. megasporophyll
C. megaspore mother cell
D. megaspore
![]() Q. 120 Taylor conducted the experiments to prove semiconservative mode of chromosome replication on
Q. 120 Taylor conducted the experiments to prove semiconservative mode of chromosome replication on
A. Vinca rosea
B. Vicia faba
C. Drosophila melanogaster
D. E. coli
![]() Q. 121 The mechanism that causes a gene to move from one linkage group to another is called
Q. 121 The mechanism that causes a gene to move from one linkage group to another is called
A. Inversion
B. Duplication
C. Translocation
D. Crossing over
![]() Q. 122 The equivalent of a structural gene is
Q. 122 The equivalent of a structural gene is
A. Mutation
B. Cistron
C. Operon
D. Recon
![]() Q. 123 A true breeding plant is
Q. 123 A true breeding plant is
A. One that is able to breed on its own
B. Produced due to cross pollination among unrelated plants
C. Near homozygous and produces offspring of its own kind
D. Always homozygous recessive in its genetic constitution
![]() Q. 124 Which of the following rRNAs acts as structural RNA as well as ribozyme in bacteria?
Q. 124 Which of the following rRNAs acts as structural RNA as well as ribozyme in bacteria?
A. 5S rRNA
B. 18S rRNA
C. 23S rRNA
D. 5.8S rRNA
![]() Q. 125 Stirred tank bioreactors have been designed for
Q. 125 Stirred tank bioreactors have been designed for
A. Purification of product
B. Addition of preservatives to the product
C. Availability of oxygen throughout the process
D. Ensuring anaerobic conditions in the culture vessel
![]() Q. 126 A foreign DNA and plasmid cut by the same restriction endonuclease can be joined to form a recombinant plasmid using
Q. 126 A foreign DNA and plasmid cut by the same restriction endonuclease can be joined to form a recombinant plasmid using
A. Eco R1
B. Taq Polymerase
C. Polymerase III
D. ligase
![]() Q. 127 Which of the following is not a component of downstream processing?
Q. 127 Which of the following is not a component of downstream processing?
A. Separation
B. Purification
C. Preservation
D. Expression
![]() Q. 128 Which of the following restriction enzymes produce blunt ends?
Q. 128 Which of the following restriction enzymes produce blunt ends?
A. Sal I
B. Eco RV
C. Xho I
D. Hind III
![]() Q. 129 Which kind of therapy was given in 1990 to a four year old girl with adenosine deaminase (ADA) deficiency?
Q. 129 Which kind of therapy was given in 1990 to a four year old girl with adenosine deaminase (ADA) deficiency?
A. Gene therapy
B. Chemotherapy
C. Immunotherapy
D. Radiation therapy
![]() Q. 130 How many hot spots of biodiversity in the world have been identified till date by Norman Myers?
Q. 130 How many hot spots of biodiversity in the world have been identified till date by Norman Myers?
A. 17
B. 25
C. 34
D. 43
![]() Q. 131 The primary producers of deep-sea hydrothermal vent ecosystem are
Q. 131 The primary producers of deep-sea hydrothermal vent ecosystem are
A. Green algae
B. Chemosynthetic bacteria
C. Blue green algae
D. Coral reefs
![]() Q. 132 Which of the following is corrent for r-selected species?
Q. 132 Which of the following is corrent for r-selected species?
A. Large number of progeny with small size
B. Large number of progeny with large size
C. Small number of progeny with small size
D. Small number of progeny with large size
![]() Q. 133 If ‘+’ sign is assigned to beneficial interactions, ‘-’ sign to detrimental and ‘0’ sign to neutral interactions, then the population interaction represented by ‘+’ ‘-’ refers to
Q. 133 If ‘+’ sign is assigned to beneficial interactions, ‘-’ sign to detrimental and ‘0’ sign to neutral interactions, then the population interaction represented by ‘+’ ‘-’ refers to
A. Mutualism
B. Amensalism
C. Commensalism
D. Parasitism
![]() Q. 134 Which of the following is correctly matched?
Q. 134 Which of the following is correctly matched?
A. Aerenchyma – Opuntia
B. Age pyramid – Biome
C. Parthenium hysterophorus – Threat to biodiversity
D. Stratification – population
![]() Q. 135 Red list contains data or information
Q. 135 Red list contains data or information
A. all economically important plants
B. Plants whose products are in international trade
C. threatened species
D. marine vertebrates only
![]() Q. 136 Which of the following sets of diseases is caused by bacteria?
Q. 136 Which of the following sets of diseases is caused by bacteria?
A. Cholera and tetanus
B. Typhoid and smallpox
C. Tetanus and mumps
D. Herpes and influenza
![]() Q. 137 Match Column-I with Column-II for housefly classification and select the correct option using the codes given below:
Q. 137 Match Column-I with Column-II for housefly classification and select the correct option using the codes given below:
A. (1)
B. (2)
C. (3)
D. (4)
![]() Q. 138 Choose the correct statement
Q. 138 Choose the correct statement
A. All mammals are viviparous
B. All cyclostomes do not possess jaws and paired fins
C. All reptiles have a three chambered heart
D. All Pisces have gills covered by an operculum
![]() Q. 139 Study the four statements (A-D) given below and select the two correct ones out of them:
Q. 139 Study the four statements (A-D) given below and select the two correct ones out of them:
A. Definition of biological species was given by Ernst Mayr
B. Photoperiod does not affect reproduction in plants
C. Binomial nomenclature system was given by R. H. Whittaker
D. In unicellular organisms, reproduction is synonymous with growth The two correct statements are
A. B and C
B. C and D
C. A and D
D. A and B
![]() Q. 140 In male cockroaches, sperms are stored in which part of the reproductive system?
Q. 140 In male cockroaches, sperms are stored in which part of the reproductive system?
A. Seminal vesicles
B. Mushroom glands
C. Testes
D. Vas deferens
![]() Q. 141 Smooth muscles are
Q. 141 Smooth muscles are
A. Involuntary, fusiform, non-striated
B. Voluntary, multinucleate, cylindrical
C. Involuntary, cylindrical, striated
D. Voluntary, spindle shaped, uninucleate
![]() Q. 142 Oxidative phosphorylation is
Q. 142 Oxidative phosphorylation is
A. Formation of ATP by transfer of phosphate group from a substrate to ADP
B. Oxidation of phosphate group in ATP
C. Addition of phosphate group to ATP
D. Formation of ATP by energy released from electrons removed during substrate oxidation
![]() Q. 143 Which of the following is the least likely to be involved in stabilizing the three dimensional folding of most proteins?
Q. 143 Which of the following is the least likely to be involved in stabilizing the three dimensional folding of most proteins?
A. Hydrogen bonds
B. Electrostatic interaction
C. Hydrophobic interaction
D. Ester bonds
![]() Q. 144 Which of the following describes the given graph correctly?
Q. 144 Which of the following describes the given graph correctly?
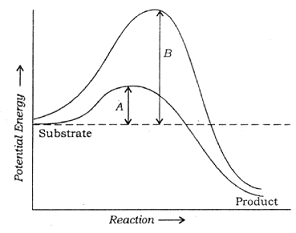
A. Endothermic reaction with energy A in presence of enzyme and B in absence of enzyme
B. Exothermic reaction with energy A in presence of enzyme and B in absence of enzyme
C. Endothermic reaction with energy A in absence of enzyme and B in presence of enzyme
D. Exothermic reaction with energy A in absence of enzyme and B in presence of enzyme
![]() Q. 145 When cell has stalled DNA replication fork, which checkpoint should be predominantly activated?
Q. 145 When cell has stalled DNA replication fork, which checkpoint should be predominantly activated?
A. G1/S
B. G2/M
C. M
D. Both G2/M and M
![]() Q. 146 Match the stages of meiosis in column I to their characteristic feature in column II and select the correct option using the codes given below:
Q. 146 Match the stages of meiosis in column I to their characteristic feature in column II and select the correct option using the codes given below:
A. (1)
B. (2)
C. (3)
D. (4)
![]() Q. 147 Which hormones do stimulate the production of pancreatic juice and bicarbonate?
Q. 147 Which hormones do stimulate the production of pancreatic juice and bicarbonate?
A. Angiotensin and epinephrine
B. Gastrin and insulin
C. Cholecystokinin and secretin
D. Insulin and Glucagon
![]() Q. 148 The partial pressure of oxygen in the alveoli of the lungs is
Q. 148 The partial pressure of oxygen in the alveoli of the lungs is
A. equal to that in the blood
B. more than that in the blood
C. less than that in the blood
D. less than that of the carbon dioxide
![]() Q. 149 Choose the correct statement.
Q. 149 Choose the correct statement.
A. Nociceptors respond to changes in pressure
B. Meissner’s corpuscles are thermoreceptors
C. Photoreceptors in the human eye are depolarized during darkness and become hyperpolarized in response to the light stimulus
D. Receptors do not produce graded potentials
![]() Q. 150 Grave’s disease is caused due to
Q. 150 Grave’s disease is caused due to
A. hyposecretion of thyroid gland
B. hypersecretion of thyroid gland
C. hyposecretion of adrenal gland
D. hypersecretion of adrenal gland
![]() Q. 151 Name the ion responsible for unmasking of active sites for myosin for cross-bridge activity during muscle contraction
Q. 151 Name the ion responsible for unmasking of active sites for myosin for cross-bridge activity during muscle contraction
A. Calcium
B. Magnesium
C. Sodium
D. Potassium
![]() Q. 152 Name the blood cells, whose reduction in number can cause clotting disorder, leading to excessive loss of blood from the body.
Q. 152 Name the blood cells, whose reduction in number can cause clotting disorder, leading to excessive loss of blood from the body.
A. Erythrocytes
B. Leucocytes
C. Neutrophils
D. Thrombocytes
![]() Q. 153 Name a peptide hormone which acts mainly on hepatocytes, adipocytes and enhances cellular glucose uptake and utilization.
Q. 153 Name a peptide hormone which acts mainly on hepatocytes, adipocytes and enhances cellular glucose uptake and utilization.
A. Insulin
B. Glucagon
C. Secretin
D. Gastrin
![]() Q. 154 Osteoporosis, age-related disease of skeletal system, may occur due to
Q. 154 Osteoporosis, age-related disease of skeletal system, may occur due to
A. immune disorder affecting neuro-muscular junction leading to fatigue
B. high concentration of Ca++ and Na+
C. decreased level of estrogen
D. accumulation of uric acid leading to inflammation of joints
![]() Q. 155 Serum differs from blood in
Q. 155 Serum differs from blood in
A. lacking globulins
B. lacking albumins
C. lacking clotting factors
D. lacking antibodies
![]() Q. 156 Lungs do not collapse between breaths and some air always remains in the lungs which can never be expelled because
Q. 156 Lungs do not collapse between breaths and some air always remains in the lungs which can never be expelled because
A. there is a negative pressure in the lungs
B. there is negative intrapleural pressure pulling at the lung walls
C. there is a positive intrapleural pressure
D. pressure in the lungs is higher than the atmospheric pressure
![]() Q. 157 The posterior pituitary gland is not a ‘true’ endocrine gland because
Q. 157 The posterior pituitary gland is not a ‘true’ endocrine gland because
A. it is provided with a duct
B. it only stores and release hormones
C. it is under the regulation of hypothalamus
D. it secretes enzymes
![]() Q. 158 The part of nephron involved in active reabsorption of sodium is
Q. 158 The part of nephron involved in active reabsorption of sodium is
A. distal convoluted tubule
B. proximal convoluted tubule
C. Bowman’s capsule
D. descending limb of Henle’s loop
![]() Q. 159 Which of the following is hormone-releasing IUD?
Q. 159 Which of the following is hormone-releasing IUD?
A. LNG-20
B. Multiload 375
C. Lippes loop
D. Cu7
![]() Q. 160 Which of following incorrect regarding vasectomy?
Q. 160 Which of following incorrect regarding vasectomy?
A. No sperm occurs in seminal fluid
B. No sperms occurs in epididymis
C. Vasa deferentia is cut and died
D. Irreversible sterility
![]() Q. 161 Embryo with more than 16 blastomeres formed due to in vitro fertilization is transferred into
Q. 161 Embryo with more than 16 blastomeres formed due to in vitro fertilization is transferred into
A. uterus
B. fallopian tube
C. fimbriae
D. cervix
![]() Q. 162 Which of the following depicts the correct pathway of transport of sperms?
Q. 162 Which of the following depicts the correct pathway of transport of sperms?
A. Rete testis → Efferent ductules → Epididymis → Vas deferens
B. Rete testis → Epididymis → Efferent ductules → Vas deferens
C. Rete testis → Vas deferens → Efferent ductules → Epididymis
D. Efferent ductules → Rete testis → Vas deferens → Epididymis
![]() Q. 163 Match Column-I with Column-II and select the correct option using the codes given below:
Q. 163 Match Column-I with Column-II and select the correct option using the codes given below:
|
Column I |
Column II |
|
a. Mons pubis |
(i) Embyo formation |
|
b. Antrum |
(ii) Sperm |
|
c. Trophectoderm |
(iii)Female external genitalia |
|
d. Nebenkern |
(iv) Graafin follicle |

A. (1)
B. (2)
C. (3)
D. (4)
![]() Q. 164 Several hormones likes hCG, hPL, estrogen, progesterone are produced by
Q. 164 Several hormones likes hCG, hPL, estrogen, progesterone are produced by
A. Ovary
B. Placenta
C. Fallopian tube
D. Pituitary
![]() Q. 165 If a colour-blind man marries a woman who is homozygous for normal vision, the probability of their son being colour blind is
Q. 165 If a colour-blind man marries a woman who is homozygous for normal vision, the probability of their son being colour blind is
A. 0
B. 0.5
C. 0.75
D. 1
![]() Q. 166 Genetic drift operates in:
Q. 166 Genetic drift operates in:
A. Small isolated population
B. Large isolated population
C. Non-reproductive population
D. Slow reproductive population
![]() Q. 167 In Hardy-Weinberg equation, the frequency of heterozygous individuals is represented by
Q. 167 In Hardy-Weinberg equation, the frequency of heterozygous individuals is represented by
A. p²
B. 2pq
C. pq
D. q²
![]() Q. 168 The chronological order of human evolution from early to the recent is
Q. 168 The chronological order of human evolution from early to the recent is
A. Australopithecus → Ramapithecus → Homo habilis → Homo erectus
B. Ramapithecus → Australopithecus → Homo habilis → Homo erectus
C. Ramapithecus → Homo habilis → Australopithecus → Homo erectus
D. Australopithecus → Homo habilis → Ramapithecus → Homo erectus
![]() Q. 169 Which of the following is the correct sequence of events in the origin of life?
Q. 169 Which of the following is the correct sequence of events in the origin of life?
I. Formation of protobionts
II. Synthesis of organic monomers
III. Synthesis of organic polymers
IV. Formation of DNA-based genetic systems
A. I, II, III, IV
B. I, III, II, IV
C. II, III, I, IV
D. II, III, IV, I
![]() Q. 170 A molecule that can act as a genetic material must fulfill the traits given below, except
Q. 170 A molecule that can act as a genetic material must fulfill the traits given below, except
A. It should be able to express itself in the form of ‘Mendelian characters’
B. It should be able to generate its replica
C. It should be unstable structurally and chemically
D. It should provide the scope for slow changes that are required for evolution
![]() Q. 171 DNA-dependent RNA polymerase catalyzes transcription on one strand of the DNA which is called the
Q. 171 DNA-dependent RNA polymerase catalyzes transcription on one strand of the DNA which is called the
A. template strand
B. coding strand
C. alpha strand
D. antistrand
![]() Q. 172 Interspecific hybridization is the mating of
Q. 172 Interspecific hybridization is the mating of
A. animals within same breed without having common ancestors
B. two different related species
C. superior males and females of different breeds
D. more closely related individuals within same breed for 4—6 generations
![]() Q. 173 Which of the following is correct regarding AIDS causative agent HIV?
Q. 173 Which of the following is correct regarding AIDS causative agent HIV?
A. HIV is enveloped virus containing one molecule of single-stranded RNA and one molecule of reverse transcriptase
B. HIV is enveloped virus that contains two identical molecules of single-stranded RNA and two molecules of reverse transcriptase
C. HIV is unenveloped virus
D. HIV does not escape but attacks the acquired immune response
![]() Q. 174 Among the following edible fishes, which one is a marine fish having rich source of omega-3 fatty acids?
Q. 174 Among the following edible fishes, which one is a marine fish having rich source of omega-3 fatty acids?
A. Mystus
B. Mangur
C. Mrigala
D. Mackerel
![]() Q. 175 Match Column-I with Column-II and select the correct option using the codes given below:
Q. 175 Match Column-I with Column-II and select the correct option using the codes given below:
Column I Column II
a. Citric acid (i) Trichoderma
b. Cyclosporin A (ii) Clostridium
c. Statins (iii) Aspergillus
d. Butyric acid (iv) Monascus
A. a – (III), b – (I), c – (II), d – (IV)
B. a – (III), b – (I), c – (IV), d – (II)
C. a – (I), b – (IV), c – (II), d – (III)
D. a – (III), b – (IV), c – (I), d – (II)
![]() Q. 176 Biochemical Oxygen Demand (BOD) may not be a good index for pollution for water bodies receiving effluents from
Q. 176 Biochemical Oxygen Demand (BOD) may not be a good index for pollution for water bodies receiving effluents from
A. Domestic sewage
B. Dairy industry
C. Petroleum industry
D. Sugar industry
![]() Q. 177 The principle of competitive exclusion was stated by
Q. 177 The principle of competitive exclusion was stated by
A. C. Darwin
B. G. F. Gause
C. MacArthur
D. Verhulst and Pearl
![]() Q. 178 Which of the following National Parks is home to the famous musk deer or hangul?
Q. 178 Which of the following National Parks is home to the famous musk deer or hangul?
A. Keibul Lamjao National Park, Manipur
B. Bandhavgarh National Park, Madhya Pradesh
C. Eaglenest Wildlife Sanctuary, Arunachal Pradesh
D. Dachigam National Park, Jammu & Kashmir
![]() Q. 179 A lake which is rich in organic waste may result in
Q. 179 A lake which is rich in organic waste may result in
A. Increased population of aquatic organisms due to minerals
B. Drying of the lake due to algal bloom
C. Increased population of fish due to lots of nutrients
D. Mortality of fish due to lack of oxygen
![]() Q. 180 The highest DDT concentration in aquatic food chain shall occur in
Q. 180 The highest DDT concentration in aquatic food chain shall occur in
A. Phytoplankton
B. Seagull
C. Crab
D. Eel
| Question | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Answer | A | D | C | A | C | B | C | C | B | B |
| Question | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Answer | B | C | B | D | B | B | D | B | B | D |
| Question | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Answer | B | D | B | C | D | C | B | B | A | C |
| Question | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 |
| Answer | D | C | A | D | C | B | B | D | A | D |
| Question | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 |
| Answer | C | D | B | A | C | C | B | A | C | B |
| Question | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 |
| Answer | A | B | D | B | C | A | D | B | A | C |
| Question | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 |
| Answer | C | B | D | C | C | B | A | C | D | C |
| Question | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 |
| Answer | D | C | B | C | B | D | A | D | C | C |
| Question | 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 | 89 | 90 |
| Answer | C | C | D | C | B | C | B | A | B | A |
| Question | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 |
| Answer | B | B | A | D | C | B | B | B | A | A |
| Question | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | 110 |
| Answer | A | C | B | B | B | A | A | D | B | B |
| Question | 111 | 112 | 113 | 114 | 115 | 116 | 117 | 118 | 119 | 120 |
| Answer | D | C | C | C | C | D | C | B | A | B |
| Question | 121 | 122 | 123 | 124 | 125 | 126 | 127 | 128 | 129 | 130 |
| Answer | C | B | C | C | C | D | D | B | A | C |
| Question | 131 | 132 | 133 | 134 | 135 | 136 | 137 | 138 | 139 | 140 |
| Answer | B | A | D | C | C | A | A | B | C | A |
| Question | 141 | 142 | 143 | 144 | 145 | 146 | 147 | 148 | 149 | 150 |
| Answer | A | D | D | B | B | A | C | B | C | B |
| Question | 151 | 152 | 153 | 154 | 155 | 156 | 157 | 158 | 159 | 160 |
| Answer | A | D | A | C | C | B | B | B | A | B |
| Question | 161 | 162 | 163 | 164 | 165 | 166 | 167 | 168 | 169 | 170 |
| Answer | A | A | B | B | A | A | B | B | C | C |
| Question | 171 | 172 | 173 | 174 | 175 | 176 | 177 | 178 | 179 | 180 |
| Answer | A | B | B | D | B | C | B | D | D | B |

